Crohn's Disease Disturbs the Immune Properties of Human Adipose-Derived Stem Cells Related to Inflammasome Activation
- PMID: 28966116
- PMCID: PMC5639166
- DOI: 10.1016/j.stemcr.2017.07.014
Crohn's Disease Disturbs the Immune Properties of Human Adipose-Derived Stem Cells Related to Inflammasome Activation
Abstract
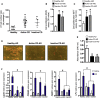
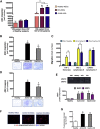
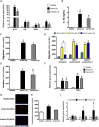
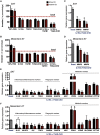
Crohn's disease (CD) is characterized by the expansion of mesenteric fat, also known as "creeping fat." We explored the plasticity and immune properties of adipose-derived stem cells (ASCs) in the context of CD as potential key players in the development of creeping fat. Mesenteric CD-derived ASCs presented a more proliferative, inflammatory, invasive, and phagocytic phenotype than equivalent cells from healthy donors, irrespective of the clinical stage. Remarkably, ASCs from the subcutaneous depot of patients with CD also showed an activated immune response that was associated with a reduction in their immunosuppressive properties. The invasive phenotype of mesenteric CD ASCs was governed by an inflammasome-mediated inflammatory state since blocking inflammasome signaling, mainly the secretion of interleukin-1β, reversed this characteristic. Thus, CD alters the biological functions of ASCs as adipocyte precursors, but also their immune properties. Selection of ASCs with the best immunomodulatory properties is advocated for the success of cell-based therapies.
Keywords: cell therapy; creeping fat; immunity; interleukin 1B; invasion; lymphocytes; mesenchymal stem cells; migration; phagocytosis; regulatory T cell.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Altundag K., Altundag O., Baptista M.Z., Akyurek S. Heparanase activity and bone loss in postmenopausal breast cancer patients. J. Clin. Oncol. 2005;23:8916–8917. author reply 8917–8918. - PubMed
-
- Batra A., Heimesaat M.M., Bereswill S., Fischer A., Glauben R., Kunkel D., Scheffold A., Erben U., Kuhl A., Loddenkemper C. Mesenteric fat - control site for bacterial translocation in colitis? Mucosal Immunol. 2012;5:580–591. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

