Peripheral Autoimmune Regulator Induces Exhaustion of CD4+ and CD8+ Effector T Cells to Attenuate Autoimmune Diabetes in Non-Obese Diabetic Mice
- PMID: 28966617
- PMCID: PMC5605615
- DOI: 10.3389/fimmu.2017.01128
Peripheral Autoimmune Regulator Induces Exhaustion of CD4+ and CD8+ Effector T Cells to Attenuate Autoimmune Diabetes in Non-Obese Diabetic Mice
Abstract
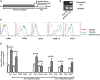
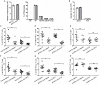
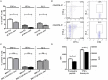
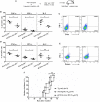
Autoimmune regulator (Aire) is one of the most crucial genes expressed in the thymus, where it orchestrates the promiscuous expression and presentation of tissue-specific antigens during thymocyte selection. The presence of Aire-expressing cells outside the thymus points toward its plausible extrathymic functions; however, the relative contribution of Aire-expressing cells of hematopoietic origin and their role in the modulation of autoimmune diseases are still obscure. Here, we report that non-obese diabetic mice with transgenic Aire expression under the control of the CD11c (integrin alpha X) promoter were significantly protected from autoimmune diabetes compared with their non-transgenic littermates. The protective effect of Aire transgene was mediated primarily by an increase in the "exhausted" populations of CD4+ and CD8+ T cells, both demonstrating poor expressions of interferon-γ and tumor necrosis factor-α. Both CD4+ and CD8+ effector T cells in transgenic mice displayed distinctive and differential expression of T-bet and Eomesodermin, respectively, in conjunction with high expression of programmed cell death protein-1 and other exhaustion-associated markers. Importantly, transgenic Aire expression did not result in any detectable changes in the population of Foxp3+ regulatory T (Treg) cells. Co-transfer experiments also demonstrated that Aire transgenic dendritic cells, as a "stand-alone" cell population, had the potential to suppress effector T cells in vivo without the support of Treg cells, but eventually failed to prevent the diabetogenesis in recipient mice. In conclusion, our study suggests that apart from its role in clonal deletion of autoreactive T cells or clonal diversion to Treg lineage, Aire can also contribute to tolerance by forcing effector T cells into a state of exhaustion with poor effector functions, thereby effectively containing autoimmune diseases.
Keywords: T cell exhaustion; autoimmune diabetes; autoimmune regulator; dendritic cells; effector T cells; regulatory T cells.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

