Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world
- PMID: 28966663
- PMCID: PMC5607928
- DOI: 10.1177/1756285617722706
Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world
Abstract
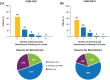
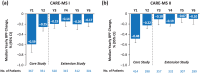
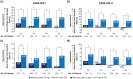
Alemtuzumab is a humanized monoclonal antibody approved for the treatment of relapsing-remitting multiple sclerosis (RRMS), given as two annual courses on five consecutive days at baseline and on three consecutive days 12 months later. Here we provide an update on the long-term efficacy and safety of alemtuzumab in RRMS, including real-world experience, and advances in our understanding of its mechanism of action. Recent data from the phase II/III extension study have demonstrated that alemtuzumab reduces relapse rates, disability worsening, and the rate of brain volume loss over the long term, with many patients achieving no evidence of disease activity. In high proportions of patients, preexisting disability remained stable or improved. Alemtuzumab is associated with a consistent safety profile over the long term, with no new safety signals emerging and the overall annual incidence of reported adverse events decreasing after the first year on treatment. Acyclovir prophylaxis reduces herpetic infections, and monitoring has been shown to mitigate the risk of autoimmune adverse events, allowing early detection and overall effective management. Data from clinical practice and ongoing observational studies are providing additional information on the real-world use of alemtuzumab. Recent evidence on the mechanism of action of alemtuzumab indicates that in addition to its previously known effects of inducing depletion and repopulation of T and B lymphocytes, it also results in a relative increase of cells with memory and regulatory phenotypes and a decrease in cells with a proinflammatory signature, and may further promote an immunoregulatory environment through an impact on other innate immune cells (e.g. dendritic cells) that play a role in MS. These effects may allow preservation of innate immunity and immunosurveillance. Together, these lines of evidence help explain the durable clinical efficacy of alemtuzumab, in the absence of continuous treatment, in patients with RRMS.
Keywords: alemtuzumab; long-term efficacy; long-term safety; mechanism of action; multiple sclerosis; real-world experience.
Conflict of interest statement
Conflict of interest statement: TZ has received consulting or speaking fees from Almirall, Bayer, Biogen, Merck, Novartis, Roche, Sanofi Genzyme, and Teva, and has received grant/research support from Biogen, Novartis, Sanofi Genzyme, and Teva. KT has received consulting fees from Biogen, Novartis, and Roche.
Figures

References
-
- Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012; 380: 1819–1828. - PubMed
-
- Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359: 1786–1801. - PubMed
-
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. - PubMed
-
- Coles AJ, Boyko AN, Cohen JA, et al. Alemtuzumab provides durable improvements in clinical outcomes in treatment-naive patients with active relapsing-remitting multiple sclerosis over 6 years in the absence of continuous treatment (CARE-MS I). Mult Scler 2016; 22: P213.
-
- Fox EJ, Alroughani R, Brassat D, et al. Efficacy of alemtuzumab is durable over 6 years in patients with active relapsing-remitting multiple sclerosis and an inadequate response to prior therapy in the absence of continuous treatment (CARE-MS II). Mult Scler 2016; 22: P1150.
LinkOut - more resources
Full Text Sources
Other Literature Sources

