Synthesis of a novel polycyclic ring scaffold with antimitotic properties via a selective domino Heck-Suzuki reaction
- PMID: 28966765
- PMCID: PMC5586250
- DOI: 10.1039/c4sc02547d
Synthesis of a novel polycyclic ring scaffold with antimitotic properties via a selective domino Heck-Suzuki reaction
Abstract
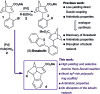
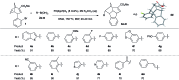
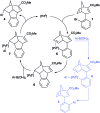
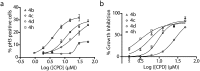
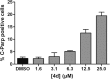
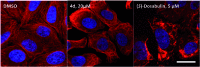
The synthesis of a previously undescribed sp3-rich 6-5-5-6 tetracyclic ring scaffold using a palladium catalysed domino Heck-Suzuki reaction is reported. This reaction is high-yielding, selective for the domino process over the direct Suzuki reaction and tolerant towards a variety of boronic acids. The novel scaffold can also be accessed via domino Heck-Stille and radical cyclisations. Compounds based around this scaffold were found to be effective antimitotic agents in a human cancer cell line. Detailed phenotypic profiling showed that the compounds affected the congression of chromosomes to give mitotic arrest and apoptotic cell death. Thus, a novel structural class of antimitotic agents that does not disrupt the tubulin network has been identified.
Figures


References
-
- Galloway W. R. J. D., Isidro-Llobet A., Spring D. R. Nat. Commun. 2010;1:80. - PubMed
- Galloway W. R. J. D. and Spring D. R., Diversity-Oriented Synthesis, 2014, vol. 1, p. 21.
- Sauer W. H., Schwarz M. K. J. Chem. Inf. Comput. Sci. 2003;43:987. - PubMed
- Dai W.-M., Diversity-Oriented Synthesis, 2013, vol. 1, p. 11.
-
-
Indeed, small molecule screening libraries that are small in size but contain multiple scaffolds are generally regarded as being superior to larger single-scaffold libraries in terms of bio-relevant diversity. See ref. 2(a) and references therein
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

