S,O-Ligand-Promoted Palladium-Catalyzed C-H Functionalization Reactions of Nondirected Arenes
- PMID: 28966841
- PMCID: PMC5617328
- DOI: 10.1021/acscatal.7b02356
S,O-Ligand-Promoted Palladium-Catalyzed C-H Functionalization Reactions of Nondirected Arenes
Abstract
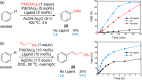
Pd(II)-catalyzed C-H functionalization of nondirected arenes has been realized using an inexpensive and easily accessible type of bidentate S,O-ligand. The catalytic system shows high efficiency in the C-H olefination reaction of electron-rich and electron-poor arenes. This methodology is operationally simple, scalable, and can be used in late-stage functionalization of complex molecules. The broad applicability of this catalyst has been showcased in other transformations such as Pd(II)-catalyzed C-H acetoxylation and allylation reactions.
Keywords: C−H activation; S,O-ligand; arenes; olefination; palladium.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
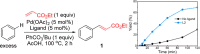

References
-
-
For general reviews on C–H functionalization, see:
- Alberico D.; Scott M. E.; Lautens M. Chem. Rev. 2007, 107, 174–238. 10.1021/cr0509760. - DOI - PubMed
- Yu J.-Q.; Shi Z.. C–H Activation; Yu J.-Q., Shi Z., Eds.; Topics in Current Chemistry, Vol. 292; Springer: Berlin, 2010. - PubMed
- Hartwig J. F. J. Am. Chem. Soc. 2016, 138, 2–24. 10.1021/jacs.5b08707. - DOI - PMC - PubMed
- Davies H. M. L.; Morton D. J. Org. Chem. 2016, 81, 343–350. 10.1021/acs.joc.5b02818. - DOI - PubMed
-
For a special issue dedicated to this topic, see:
- Doyle M. P.; Goldberg K. I.. Acc. Chem. Res. 2012, 45, 777.10.1021/ar300096z - DOI - PubMed
-
-
- Lyons T. W.; Sanford M. S. Chem. Rev. 2010, 110, 1147–1169. 10.1021/cr900184e. - DOI - PMC - PubMed
- Chen Z.; Wang B.; Zhang J.; Yu W.; Liu Z.; Zhang Y. Org. Chem. Front. 2015, 2, 1107–1295. 10.1039/C5QO00004A. - DOI
- Maes J.; Maes B. U. W. Adv. Heterocycl. Chem. 2016, 120, 137–194. 10.1016/bs.aihch.2016.04.005. - DOI
- Pototschnig G.; Maulide N.; Schnürch M. Chem.—Eur. J. 2017, 23, 9206–9232. 10.1002/chem.201605657. - DOI - PubMed
- Ma W.; Gandeepan P.; Li J.; Ackermann L. Org. Chem. Front. 2017, 4, 1435–1467. 10.1039/C7QO00134G. - DOI
-
-
For a review on C–H functionalization of substrates that do not bear directing groups, see:
- Kuhl N.; Hopkinson M. N.; Wencel-Delord J.; Glorius F. Angew. Chem., Int. Ed. 2012, 51, 10236–10254. 10.1002/anie.201203269. - DOI - PubMed
-
For selected examples, see:
- Robbins D. W.; Hartwig J. F. Angew. Chem., Int. Ed. 2013, 52, 933–937. 10.1002/anie.201208203. - DOI - PubMed
- Shrestha R.; Mukherjee P.; Tan Y.; Litman Z. C.; Hartwig J. F. J. Am. Chem. Soc. 2013, 135, 8480–8483. 10.1021/ja4032677. - DOI - PubMed
- Ricci P.; Krämer K.; Larrosa I. J. Am. Chem. Soc. 2014, 136, 18082–18086. 10.1021/ja510260j. - DOI - PMC - PubMed
- Cheng C.; Hartwig J. F. J. Am. Chem. Soc. 2015, 137, 592–595. 10.1021/ja511352u. - DOI - PubMed
- Larsen M. A.; Wilson C. V.; Hartwig J. F. J. Am. Chem. Soc. 2015, 137, 8633–8643. 10.1021/jacs.5b04899. - DOI - PubMed
- Paudyal M. P.; Adebesin A. M.; Burt S. R.; Ess D. H.; Ma Z.; Kürti L.; Falck J. R. Science 2016, 353, 1144–1147. 10.1126/science.aaf8713. - DOI - PMC - PubMed
- Luan Y.-X.; Zhang T.; Yao W.-W.; Lu K.; Kong L.-Y.; Lin Y.-T.; Ye M. J. Am. Chem. Soc. 2017, 139, 1786–1789. 10.1021/jacs.6b12907. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
