Hyperspectral imaging with laser-scanning sum-frequency generation microscopy
- PMID: 28966861
- PMCID: PMC5611937
- DOI: 10.1364/BOE.8.004230
Hyperspectral imaging with laser-scanning sum-frequency generation microscopy
Abstract
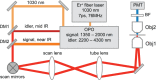
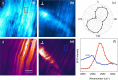
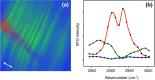
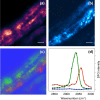
Vibrationally sensitive sum-frequency generation (SFG) microscopy is a chemically selective imaging technique sensitive to non-centrosymmetric molecular arrangements in biological samples. The routine use of SFG microscopy has been hampered by the difficulty of integrating the required mid-infrared excitation light into a conventional, laser-scanning nonlinear optical (NLO) microscope. In this work, we describe minor modifications to a regular laser-scanning microscope to accommodate SFG microscopy as an imaging modality. We achieve vibrationally sensitive SFG imaging of biological samples with sub-μm resolution at image acquisition rates of 1 frame/s, almost two orders of magnitude faster than attained with previous point-scanning SFG microscopes. Using the fast scanning capability, we demonstrate hyperspectral SFG imaging in the CH-stretching vibrational range and point out its use in the study of molecular orientation and arrangement in biologically relevant samples. We also show multimodal imaging by combining SFG microscopy with second-harmonic generation (SHG) and coherent anti-Stokes Raman scattering (CARS) on the same imaging platfrom. This development underlines that SFG microscopy is a unique modality with a spatial resolution and image acquisition time comparable to that of other NLO imaging techniques, making point-scanning SFG microscopy a valuable member of the NLO imaging family.
Keywords: (110.3080) Infrared imaging; (170.0180) Microscopy; (180.4315) Nonlinear microscopy; (190.4223) Nonlinear wave mixing.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures
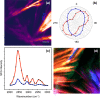
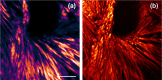
References
-
- Masters B. R., So P. T. C., Handbook of Biomedical Nonlinear Optical Microscopy (Oxford University Press, Oxford, 2008)
-
- Pavone F. S., Campagnola P. J., Second Harmonic Generation Imaging (CRC Press, Boca Raton, 2013).
-
- Cheng J. X., Xie X. S., Coherent Raman Scattering Microscopy (CRC Press, Boca Raton, 2013).
-
- Shen Y. R., “Surface properties probed by second-harmonic and sum-frequency generation,” Nature 337, 519–525 (1989).10.1038/337519a0 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
