Rapid magnetic isolation of extracellular vesicles via lipid-based nanoprobes
- PMID: 28966872
- PMCID: PMC5618714
- DOI: 10.1038/s41551-017-0058
Rapid magnetic isolation of extracellular vesicles via lipid-based nanoprobes
Abstract
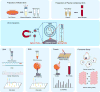
Extracellular vesicles (EVs) can mediate intercellular communication by transferring cargo proteins and nucleic acids between cells. The pathophysiological roles and clinical value of EVs are under intense investigation, yet most studies are limited by technical challenges in the isolation of nanoscale EVs (nEVs). Here, we report a lipid nanoprobe that enables spontaneous labelling and magnetic enrichment of nEVs in 15 minutes, with isolation efficiency and cargo composition similar to what can be achieved by the much slower and bulkier method of ultracentrifugation. We also show that the lipid nanoprobes, which allow for downstream analyses of nucleic acids and proteins, enabled the identification of EGFR and KRAS mutations following nEV isolation from blood plasma from non-small-cell lung-cancer patients. The efficiency and versatility of the lipid nanoprobe opens up opportunities in point-of-care cancer diagnostics.
Conflict of interest statement
Competing interests The authors declare no competing financial interests.
Figures



Similar articles
-
Enrichment of extracellular vesicles with lipid nanoprobe functionalized nanostructured silica.Lab Chip. 2019 Jul 9;19(14):2346-2355. doi: 10.1039/c8lc01359d. Lab Chip. 2019. PMID: 31232418 Free PMC article.
-
Affinity-Based Enrichment of Extracellular Vesicles with Lipid Nanoprobes.Methods Mol Biol. 2022;2394:185-197. doi: 10.1007/978-1-0716-1811-0_12. Methods Mol Biol. 2022. PMID: 35094329
-
Rapid Capture and Nondestructive Release of Extracellular Vesicles Using Aptamer-Based Magnetic Isolation.ACS Sens. 2019 May 24;4(5):1245-1251. doi: 10.1021/acssensors.9b00060. Epub 2019 Apr 29. ACS Sens. 2019. PMID: 30915846
-
Cellular communication through extracellular vesicles and lipid droplets.J Extracell Biol. 2023 Mar 1;2(3):e77. doi: 10.1002/jex2.77. eCollection 2023 Mar. J Extracell Biol. 2023. PMID: 38938415 Free PMC article. Review.
-
Opportunities and challenges of natural killer cell-derived extracellular vesicles.Front Bioeng Biotechnol. 2023 Mar 31;11:1122585. doi: 10.3389/fbioe.2023.1122585. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37064251 Free PMC article. Review.
Cited by
-
Circulating Exosomal miR-96 as a Novel Biomarker for Radioresistant Non-Small-Cell Lung Cancer.J Oncol. 2021 Feb 27;2021:5893981. doi: 10.1155/2021/5893981. eCollection 2021. J Oncol. 2021. PMID: 33727921 Free PMC article.
-
Extracellular vesicle-based liquid biopsy biomarkers and their application in precision immuno-oncology.Biomark Res. 2023 Nov 17;11(1):99. doi: 10.1186/s40364-023-00540-2. Biomark Res. 2023. PMID: 37978566 Free PMC article. Review.
-
Conferring receptors on recipient cells with extracellular vesicles for targeted drug delivery.Bioact Mater. 2020 Sep 24;6(3):749-756. doi: 10.1016/j.bioactmat.2020.09.016. eCollection 2021 Mar. Bioact Mater. 2020. PMID: 33024896 Free PMC article.
-
Future of Digital Assays to Resolve Clinical Heterogeneity of Single Extracellular Vesicles.ACS Nano. 2022 Aug 23;16(8):11619-11645. doi: 10.1021/acsnano.2c04337. Epub 2022 Jul 29. ACS Nano. 2022. PMID: 35904433 Free PMC article. Review.
-
Extracellular vesicle-based EGFR genotyping in bronchoalveolar lavage fluid.Transl Lung Cancer Res. 2020 Apr;9(2):168-171. doi: 10.21037/tlcr.2020.03.06. Transl Lung Cancer Res. 2020. PMID: 32420055 Free PMC article. No abstract available.
References
-
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. - PubMed
-
- Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 25:364–372. - PubMed
-
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

