Challenges and Opportunities with Predicting in Vivo Phase II Metabolism via Glucuronidation from in Vitro Data
- PMID: 28966903
- PMCID: PMC5613675
- DOI: 10.1007/s40495-016-0076-8
Challenges and Opportunities with Predicting in Vivo Phase II Metabolism via Glucuronidation from in Vitro Data
Abstract
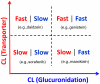
Glucuronidation is the most important phase II metabolic pathway which is responsible for the clearance of many endogenous and exogenous compounds. To better understand the elimination process for compounds undergoing glucuronidation and identify compounds with desirable in vivo pharmacokinetic properties, many efforts have been made to predict in vivo glucuronidation using in vitro data. In this article, we reviewed typical approaches used in previous predictions. The problems and challenges in prediction of glucuronidation were discussed. Besides that different incubation conditions can affect the prediction accuracy, other factors including efflux / uptake transporters, enterohepatic recycling, and deglucuronidation reactions also contribute to the disposition of glucuronides and make the prediction more difficult. PBPK modeling, which can describe more complicated process in vivo, is a promising prediction strategy which may greatly improve the prediction of glucuronidation and potential DDIs involving glucuronidation. Based on previous studies, we proposed a transport-glucuronidation classification system, which was built based on the kinetics of both glucuronidation and transport of the glucuronide. This system could be a very useful tool to achieve better in vivo predictions.
Keywords: Glucuronidation; PBPK; Prediction; Transport-Glucuronidation Classification System; Transporter.
Figures
Similar articles
-
Transport-Glucuronidation Classification System and PBPK Modeling: New Approach To Predict the Impact of Transporters on Disposition of Glucuronides.Mol Pharm. 2017 Sep 5;14(9):2884-2898. doi: 10.1021/acs.molpharmaceut.6b00941. Epub 2017 Mar 13. Mol Pharm. 2017. PMID: 28221813
-
Decreased Expression of Multidrug Resistance-Associated Protein 4 (MRP4/ABCC4) Leads to Reduced Glucuronidation of Flavonoids in UGT1A1-Overexpressing HeLa Cells: The Role of Futile Recycling.J Agric Food Chem. 2015 Jul 8;63(26):6001-8. doi: 10.1021/acs.jafc.5b00983. Epub 2015 Jun 22. J Agric Food Chem. 2015. PMID: 26066637
-
Interplay of Efflux Transporters with Glucuronidation and Its Impact on Subcellular Aglycone and Glucuronide Disposition: A Case Study with Kaempferol.Mol Pharm. 2018 Dec 3;15(12):5602-5614. doi: 10.1021/acs.molpharmaceut.8b00782. Epub 2018 Nov 9. Mol Pharm. 2018. PMID: 30376625
-
Quantitative prediction of glucuronidation in humans using the in vitro- in vivo extrapolation approach.Curr Top Med Chem. 2013;13(11):1343-52. doi: 10.2174/15680266113139990038. Curr Top Med Chem. 2013. PMID: 23675940 Review.
-
Dealing with the complex drug-drug interactions: towards mechanistic models.Biopharm Drug Dispos. 2015 Mar;36(2):71-92. doi: 10.1002/bdd.1934. Epub 2015 Feb 10. Biopharm Drug Dispos. 2015. PMID: 25545151 Review.
Cited by
-
Defining the Role of GLI/Hedgehog Signaling in Chemoresistance: Implications in Therapeutic Approaches.Cancers (Basel). 2021 Sep 23;13(19):4746. doi: 10.3390/cancers13194746. Cancers (Basel). 2021. PMID: 34638233 Free PMC article. Review.
-
In Silico Analyses of a Promising Drug Candidate for the Treatment of Amyotrophic Lateral Sclerosis Targeting Superoxide Dismutase I Protein.Pharmaceutics. 2023 Mar 29;15(4):1095. doi: 10.3390/pharmaceutics15041095. Pharmaceutics. 2023. PMID: 37111580 Free PMC article.
-
Untargeted MSn-Based Monitoring of Glucuronides in Fish: Screening Complex Mixtures for Contaminants with Biological Relevance.ACS ES T Water. 2022 Dec 9;2(12):2481-2490. doi: 10.1021/acsestwater.2c00310. ACS ES T Water. 2022. PMID: 37288388 Free PMC article.
-
Racial Disparity in Drug Disposition in the Digestive Tract.Int J Mol Sci. 2021 Jan 21;22(3):1038. doi: 10.3390/ijms22031038. Int J Mol Sci. 2021. PMID: 33494365 Free PMC article. Review.
-
Evaluation of Clinically Relevant Drug-Drug Interactions and Population Pharmacokinetics of Darolutamide in Patients with Nonmetastatic Castration-Resistant Prostate Cancer: Results of Pre-Specified and Post Hoc Analyses of the Phase III ARAMIS Trial.Target Oncol. 2019 Oct;14(5):527-539. doi: 10.1007/s11523-019-00674-0. Target Oncol. 2019. PMID: 31571095 Free PMC article. Clinical Trial.
References
-
- Kolaand I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nature reviews Drug discovery. 2004;3:711–715. - PubMed
-
- Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, Peterkin V, Koup JR, Ball SE. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug metabolism and disposition: the biological fate of chemicals. 2004;32:1201–1208. - PubMed
-
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenetics and genomics. 2005;15:677–685. - PubMed
-
- Miners JO, Mackenzie PI, Knights KM. The prediction of drug-glucuronidation parameters in humans: UDP-glucuronosyltransferase enzyme-selective substrate and inhibitor probes for reaction phenotyping and in vitro-in vivo extrapolation of drug clearance and drug-drug interaction potential. Drug metabolism reviews. 2010;42:196–208. - PubMed
-
- Naritomi Y, Nakamori F, Furukawa T, Tabata K. Prediction of hepatic and intestinal glucuronidation using in vitro-in vivo extrapolation. Drug metabolism and pharmacokinetics. 2015;30:21–29. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
