Subthalamic Nucleus Deep Brain Stimulation: Basic Concepts and Novel Perspectives
- PMID: 28966978
- PMCID: PMC5617209
- DOI: 10.1523/ENEURO.0140-17.2017
Subthalamic Nucleus Deep Brain Stimulation: Basic Concepts and Novel Perspectives
Abstract
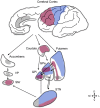
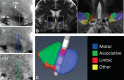
Over the last decades, extensive basic and clinical knowledge has been acquired on the use of subthalamic nucleus (STN) deep brain stimulation (DBS) for Parkinson's disease (PD). It is now clear that mechanisms involved in the effects of this therapy are far more complex than previously anticipated. At frequencies commonly used in clinical practice, neural elements may be excited or inhibited and novel dynamic states of equilibrium are reached. Electrode contacts used for chronic DBS in PD are placed near the dorsal border of the nucleus, a highly cellular region. DBS may thus exert its effects by modulating these cells, hyperdirect projections from motor cortical areas, afferent and efferent fibers to the motor STN. Advancements in neuroimaging techniques may allow us to identify these structures optimizing surgical targeting. In this review, we provide an update on mechanisms and the neural elements modulated by STN DBS.
Keywords: anatomy; deep brain stimulation; mechanisms; neuroimaging; physiology; plasticity; subthalamic nucleus.
Figures




Similar articles
-
Subthalamic nucleus deep brain stimulation for Parkinson's disease: current trends and future directions.Expert Rev Med Devices. 2020 Oct;17(10):1063-1074. doi: 10.1080/17434440.2020.1747433. Epub 2020 Apr 6. Expert Rev Med Devices. 2020. PMID: 32250645 Review.
-
Cortical Plasticity Induction by Pairing Subthalamic Nucleus Deep-Brain Stimulation and Primary Motor Cortical Transcranial Magnetic Stimulation in Parkinson's Disease.J Neurosci. 2016 Jan 13;36(2):396-404. doi: 10.1523/JNEUROSCI.2499-15.2016. J Neurosci. 2016. PMID: 26758832 Free PMC article.
-
Improving outcomes of subthalamic nucleus deep brain stimulation in Parkinson's disease.Expert Rev Neurother. 2015 Oct;15(10):1151-60. doi: 10.1586/14737175.2015.1081815. Epub 2015 Sep 17. Expert Rev Neurother. 2015. PMID: 26377740 Review.
-
Speech changes induced by deep brain stimulation of the subthalamic nucleus in Parkinson disease: involvement of the dentatorubrothalamic tract.J Neurosurg. 2017 Jun;126(6):2017-2027. doi: 10.3171/2016.5.JNS16243. Epub 2016 Sep 9. J Neurosurg. 2017. PMID: 27611200
-
Stimulation sites in the subthalamic nucleus and clinical improvement in Parkinson's disease: a new approach for active contact localization.J Neurosurg. 2016 Nov;125(5):1068-1079. doi: 10.3171/2015.9.JNS15868. Epub 2016 Feb 5. J Neurosurg. 2016. PMID: 26848922
Cited by
-
Deep Brain Stimulation in the Subthalamic Nucleus Can Improve Skilled Forelimb Movements and Retune Dynamics of Striatal Networks in a Rat Stroke Model.Int J Mol Sci. 2022 Dec 14;23(24):15862. doi: 10.3390/ijms232415862. Int J Mol Sci. 2022. PMID: 36555504 Free PMC article.
-
Deep Brain Stimulation and L-DOPA Therapy: Concepts of Action and Clinical Applications in Parkinson's Disease.Front Neurol. 2018 Aug 27;9:711. doi: 10.3389/fneur.2018.00711. eCollection 2018. Front Neurol. 2018. PMID: 30210436 Free PMC article. Review.
-
Somatotopic Organization of Hyperdirect Pathway Projections From the Primary Motor Cortex in the Human Brain.Front Neurol. 2022 Apr 25;13:791092. doi: 10.3389/fneur.2022.791092. eCollection 2022. Front Neurol. 2022. PMID: 35547388 Free PMC article.
-
Pacemaker and Defibrillator Implantation and Programming in Patients with Deep Brain Stimulation.Arrhythm Electrophysiol Rev. 2019 May;8(2):138-142. doi: 10.15420/aer.2018.63.2. Arrhythm Electrophysiol Rev. 2019. PMID: 31114689 Free PMC article. Review.
-
Altered sleep intensity upon DBS to hypothalamic sleep-wake centers in rats.Transl Neurosci. 2021 Dec 31;12(1):611-625. doi: 10.1515/tnsci-2020-0202. eCollection 2021 Jan 1. Transl Neurosci. 2021. PMID: 35070444 Free PMC article.
References
-
- Alexander GE, Crutcher MD, DeLong MR (1990) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, "prefrontal" and "limbic" functions. Prog Brain Res 85:119–146. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
