Epigenetic regulation of melatonin receptors in neuropsychiatric disorders
- PMID: 28967098
- PMCID: PMC6057907
- DOI: 10.1111/bph.14058
Epigenetic regulation of melatonin receptors in neuropsychiatric disorders
Abstract
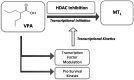
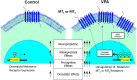
Melatonin, the primary indoleamine hormone of the mammalian pineal gland, is known to have a plethora of neuroregulatory, neuroprotective and other properties. Melatonergic signalling is mediated by its two GPCRs, MT1 and MT2 , which are widely expressed in the mammalian CNS. Melatonin levels and receptor expression often show a decrease during normal ageing, and this reduction may be accelerated in some disease states. Depleted melatonergic signalling has been associated with neuropsychiatric dysfunction and impairments in cognition, memory, neurogenesis and neurorestorative processes. The anticonvulsant and mood stabilizer, valproic acid (VPA), up-regulates melatonin MT1 and/or MT2 receptor expression in cultured cells and in the rat brain. VPA is known to affect gene expression through several mechanisms, including the modulation of intracellular kinase pathways and transcription factors, as well as the inhibition of histone deacetylase (HDAC) activity. Interestingly, other HDAC inhibitors, such as trichostatin A, which are structurally distinct from VPA, can also up-regulate melatonin receptor expression, unlike a VPA analogue, valpromide, which lacks HDAC inhibitory activity. Moreover, VPA increases histone H3 acetylation along the length of the MT1 gene promoter in rat C6 cells. These findings indicate that an epigenetic mechanism, linked to histone hyperacetylation/chromatin remodelling and associated changes in gene transcription, is involved in the up-regulation of melatonin receptors by VPA. Epigenetic induction of MT1 and/or MT2 receptor expression, in areas where these receptors are lost because of ageing, injury or disease, may be a promising therapeutic avenue for the management of CNS dysfunction and other disorders. LINKED ARTICLES: This article is part of a themed section on Recent Developments in Research of Melatonin and its Potential Therapeutic Applications. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.16/issuetoc.
© 2017 The British Pharmacological Society.
Figures

Similar articles
-
5-Azacytidine upregulates melatonin MT1 receptor expression in rat C6 glioma cells: oncostatic implications.Mol Biol Rep. 2020 Jun;47(6):4867-4873. doi: 10.1007/s11033-020-05482-8. Epub 2020 May 14. Mol Biol Rep. 2020. PMID: 32410138
-
Epigenetic induction of melatonin MT1 receptors by valproate: Neurotherapeutic implications.Eur Neuropsychopharmacol. 2017 Aug;27(8):828-832. doi: 10.1016/j.euroneuro.2017.06.002. Epub 2017 Jun 22. Eur Neuropsychopharmacol. 2017. PMID: 28648552
-
Regional upregulation of hippocampal melatonin MT2 receptors by valproic acid: therapeutic implications for Alzheimer's disease.Neurosci Lett. 2014 Jul 25;576:84-7. doi: 10.1016/j.neulet.2014.05.056. Epub 2014 Jun 5. Neurosci Lett. 2014. PMID: 24909617
-
Melatonin, Melatonin Receptors and Sleep: Moving Beyond Traditional Views.J Pineal Res. 2024 Oct;76(7):e13011. doi: 10.1111/jpi.13011. J Pineal Res. 2024. PMID: 39400423 Review.
-
Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology.J Psychiatry Neurosci. 2014 Jan;39(1):6-21. doi: 10.1503/jpn.130009. J Psychiatry Neurosci. 2014. PMID: 23971978 Free PMC article. Review.
Cited by
-
Melatonin and Melatonergic Influence on Neuronal Transcription Factors: Implications for the Development of Novel Therapies for Neurodegenerative Disorders.Curr Neuropharmacol. 2020;18(7):563-577. doi: 10.2174/1570159X18666191230114339. Curr Neuropharmacol. 2020. PMID: 31885352 Free PMC article. Review.
-
The morphological and functional characteristics of the pineal gland.Med Pharm Rep. 2019 Jul;92(3):226-234. doi: 10.15386/mpr-1235. Epub 2019 Jul 31. Med Pharm Rep. 2019. PMID: 31460502 Free PMC article. Review.
-
Pineal gland dysfunction in Alzheimer's disease: relationship with the immune-pineal axis, sleep disturbance, and neurogenesis.Mol Neurodegener. 2019 Jul 11;14(1):28. doi: 10.1186/s13024-019-0330-8. Mol Neurodegener. 2019. PMID: 31296240 Free PMC article. Review.
-
Exploring melatonin's multifaceted role in female reproductive health: From follicular development to lactation and its therapeutic potential in obstetric syndromes.J Adv Res. 2025 Apr;70:223-242. doi: 10.1016/j.jare.2024.04.025. Epub 2024 Apr 30. J Adv Res. 2025. PMID: 38692429 Free PMC article. Review.
-
5-Azacytidine upregulates melatonin MT1 receptor expression in rat C6 glioma cells: oncostatic implications.Mol Biol Rep. 2020 Jun;47(6):4867-4873. doi: 10.1007/s11033-020-05482-8. Epub 2020 May 14. Mol Biol Rep. 2020. PMID: 32410138
References
-
- Adi N, Mash DC, Ali Y, Singer C, Shehadeh L, Papapetropoulos S (2010). Melatonin MT1 and MT2 receptor expression in Parkinson's disease. Med Sci Monit 16: BR61–BR67. - PubMed
-
- Ali T, Kim MO (2015). Melatonin ameliorates amyloid beta‐induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus. J Pineal Res 59: 47–59. - PubMed
-
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013). GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138: 155–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

