Antidepressant-like effects of BU10119, a novel buprenorphine analogue with mixed κ/μ receptor antagonist properties, in mice
- PMID: 28967123
- PMCID: PMC6016679
- DOI: 10.1111/bph.14060
Antidepressant-like effects of BU10119, a novel buprenorphine analogue with mixed κ/μ receptor antagonist properties, in mice
Abstract
Background and purpose: The κ receptor antagonists have potential for treating neuropsychiatric disorders. We have investigated the in vivo pharmacology of a novel buprenorphine analogue, BU10119, for the first time.
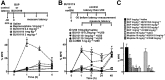
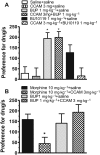
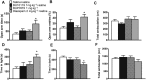
Experimental approach: To determine the opioid pharmacology of BU10119 (0.3-3 mg·kg-1 , i.p.) in vivo, the warm-water tail-withdrawal assay was applied in adult male CD1 mice. A range of behavioural paradigms was used to investigate the locomotor effects, rewarding properties and antidepressant or anxiolytic potential of BU10119. Additional groups of mice were exposed to a single (1 × 2 h) or repeated restraint stress (3× daily 2 h) to determine the ability of BU10119 to block stress-induced analgesia.
Key results: BU10119 alone was without any antinociceptive activity. BU10119 (1 mg·kg-1 ) was able to block U50,488, buprenorphine and morphine-induced antinociception. The κ antagonist effects of BU10119 in the tail-withdrawal assay reversed between 24 and 48 h. BU10119 was without significant locomotor or rewarding effects. BU10119 (1 mg·kg-1 ) significantly reduced the latency to feed in the novelty-induced hypophagia task and reduced immobility time in the forced swim test, compared to saline-treated animals. There were no significant effects of BU10119 in either the elevated plus maze or the light-dark box. Both acute and repeated restraint stress-induced analgesia were blocked by pretreatment with BU10119 (1 mg·kg-1 ). Parallel stress-induced increases in plasma corticosterone were not affected.
Conclusions and implications: BU10119 is a mixed κ/μ receptor antagonist with relatively short-duration κ antagonist activity. Based on these preclinical data, BU10119 has therapeutic potential for the treatment of depression and other stress-induced conditions.
Linked articles: This article is part of a themed section on Emerging Areas of Opioid Pharmacology. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.14/issuetoc.
© 2017 The British Pharmacological Society.
Figures



References
-
- Allen CP, Zhou Y, Leri F (2013). Effect of food restriction on cocaine locomotor sensitization in Sprague–Dawley rats. Psychopharmacology (Berl) 226: 571–578. - PubMed
-
- Botelho AP, Gameiro GH, Tuma CEDSN, Marcondes FK, Ferraz De Arruda Veiga MC (2010). The effects of acute restraint stress on nociceptive responses evoked by the injection of formalin into the temporomandibular joint of female rats. Stress 13: 269–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

