Combining Constitutively Active Rheb Expression and Chondroitinase Promotes Functional Axonal Regeneration after Cervical Spinal Cord Injury
- PMID: 28967557
- PMCID: PMC5768590
- DOI: 10.1016/j.ymthe.2017.08.011
Combining Constitutively Active Rheb Expression and Chondroitinase Promotes Functional Axonal Regeneration after Cervical Spinal Cord Injury
Abstract
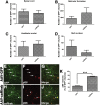
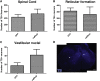
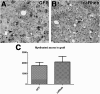
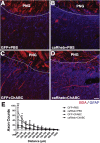
After spinal cord injury (SCI), severed axons in the adult mammalian CNS are unable to mount a robust regenerative response. In addition, the glial scar at the lesion site further restricts the regenerative potential of axons. We hypothesized that a combinatorial approach coincidentally targeting these obstacles would promote axonal regeneration. We combined (1) transplantation of a growth-permissive peripheral nerve graft (PNG) into an incomplete, cervical lesion cavity; (2) transduction of neurons rostral to the SCI site to express constitutively active Rheb (caRheb; a Ras homolog enriched in brain), a GTPase that directly activates the growth-promoting pathway mammalian target of rapamycin (mTOR) via AAV-caRheb injection; and (3) digestion of growth-inhibitory chondroitin sulfate proteoglycans within the glial scar at the distal PNG interface using the bacterial enzyme chondroitinase ABC (ChABC). We found that expressing caRheb in neurons post-SCI results in modestly yet significantly more axons regenerating across a ChABC-treated distal graft interface into caudal spinal cord than either treatment alone. Excitingly, we found that caRheb+ChABC treatment significantly potentiates the formation of synapses in the host spinal cord and improves the animals' ability to use the affected forelimb. Thus, this combination strategy enhances functional axonal regeneration following a cervical SCI.
Keywords: Rheb; axon regeneration; chondroitinase; mTOR; peripheral nerve graft; spinal cord injury.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Expressing Constitutively Active Rheb in Adult Neurons after a Complete Spinal Cord Injury Enhances Axonal Regeneration beyond a Chondroitinase-Treated Glial Scar.J Neurosci. 2015 Aug 5;35(31):11068-80. doi: 10.1523/JNEUROSCI.0719-15.2015. J Neurosci. 2015. PMID: 26245968 Free PMC article.
-
Pharmacologically inhibiting kinesin-5 activity with monastrol promotes axonal regeneration following spinal cord injury.Exp Neurol. 2015 Jan;263:172-6. doi: 10.1016/j.expneurol.2014.10.013. Epub 2014 Oct 24. Exp Neurol. 2015. PMID: 25447935 Free PMC article.
-
Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site.Exp Neurol. 2013 Jan;239:91-100. doi: 10.1016/j.expneurol.2012.09.011. Epub 2012 Sep 27. Exp Neurol. 2013. PMID: 23022460 Free PMC article.
-
Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury.Brain Res Bull. 2011 Mar 10;84(4-5):306-16. doi: 10.1016/j.brainresbull.2010.06.015. Epub 2010 Jul 8. Brain Res Bull. 2011. PMID: 20620201 Review.
-
Spinal cord regeneration.Cell Transplant. 2014;23(4-5):573-611. doi: 10.3727/096368914X678427. Cell Transplant. 2014. PMID: 24816452 Review.
Cited by
-
Progesterone attenuates neurological deficits and exerts a protective effect on damaged axons via the PI3K/AKT/mTOR-dependent pathway in a mouse model of intracerebral hemorrhage.Aging (Albany NY). 2022 Mar 19;14(6):2574-2589. doi: 10.18632/aging.203954. Epub 2022 Mar 19. Aging (Albany NY). 2022. PMID: 35305084 Free PMC article.
-
Bridging the gap of axonal regeneration in the central nervous system: A state of the art review on central axonal regeneration.Front Neurosci. 2022 Nov 9;16:1003145. doi: 10.3389/fnins.2022.1003145. eCollection 2022. Front Neurosci. 2022. PMID: 36440273 Free PMC article. Review.
-
Knockdown of Fidgetin Improves Regeneration of Injured Axons by a Microtubule-Based Mechanism.J Neurosci. 2019 Mar 13;39(11):2011-2024. doi: 10.1523/JNEUROSCI.1888-18.2018. Epub 2019 Jan 15. J Neurosci. 2019. PMID: 30647150 Free PMC article.
-
AKT3 Gene Transfer Promotes Anabolic Reprogramming and Photoreceptor Neuroprotection in a Pre-clinical Model of Retinitis Pigmentosa.Mol Ther. 2019 Jul 3;27(7):1313-1326. doi: 10.1016/j.ymthe.2019.04.009. Epub 2019 Apr 14. Mol Ther. 2019. PMID: 31043342 Free PMC article.
-
Co-targeting myelin inhibitors and CSPGs markedly enhances regeneration of GDNF-stimulated, but not conditioning-lesioned, sensory axons into the spinal cord.Elife. 2021 May 4;10:e63050. doi: 10.7554/eLife.63050. Elife. 2021. PMID: 33942723 Free PMC article.
References
-
- Schwab M.E., Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996;76:319–370. - PubMed
-
- Liu K., Tedeschi A., Park K.K., He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 2011;34:131–152. - PubMed
-
- Ye J.H., Houle J.D. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp. Neurol. 1997;143:70–81. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

