Intravenous immunoglobulin for the treatment of childhood encephalitis
- PMID: 28967695
- PMCID: PMC6485509
- DOI: 10.1002/14651858.CD011367.pub2
Intravenous immunoglobulin for the treatment of childhood encephalitis
Abstract
Background: Encephalitis is a syndrome of neurological dysfunction due to inflammation of the brain parenchyma, caused by an infection or an exaggerated host immune response, or both. Attenuation of brain inflammation through modulation of the immune response could improve patient outcomes. Biological agents such as immunoglobulin that have both anti-inflammatory and immunomodulatory properties may therefore be useful as adjunctive therapies for people with encephalitis.
Objectives: To assess the efficacy and safety of intravenous immunoglobulin (IVIG) as add-on treatment for children with encephalitis.
Search methods: The Cochrane Multiple Sclerosis and Rare Diseases of the CNS group's Information Specialist searched the following databases up to 30 September 2016: CENTRAL, MEDLINE, Embase, CINAHL, ClinicalTrials.gov, and the WHO ICTRP Search Portal. In addition, two review authors searched Science Citation Index Expanded (SCI-EXPANDED) & Conference Proceedings Citation Index - Science (CPCI-S) (Web of Science Core Collection, Thomson Reuters) (1945 to January 2016), Global Health Library (Virtual Health Library), and Database of Abstracts of Reviews of Effects (DARE).
Selection criteria: Randomised controlled trials (RCTs) comparing IVIG in addition to standard care versus standard care alone or placebo.
Data collection and analysis: Two review authors independently selected articles for inclusion, extracted relevant data, and assessed quality of trials. We resolved disagreements by discussion among the review authors. Where possible, we contacted authors of included studies for additional information. We presented results as risk ratios (RR) or mean differences (MD) with 95% confidence intervals (CI).
Main results: The search identified three RCTs with 138 participants. All three trials included only children with viral encephalitis, one of these included only children with Japanese encephalitis, a specific form of viral encephalitis. Only the trial of Japanese encephalitis (22 children) contributed to the primary outcome of this review and follow-up in that study was for three to six months after hospital discharge. There was no follow-up of participants in the other two studies. We identified one ongoing trial.For the primary outcomes, the results showed no significant difference between IVIG and placebo when used in the treatment of children with Japanese encephalitis: significant disability (RR 0.75, 95% CI 0.22 to 2.60; P = 0.65) and serious adverse events (RR 1.00, 95% CI 0.07 to 14.05; P = 1.00).For the secondary outcomes, the study of Japanese encephalitis showed no significant difference between IVIG and placebo when assessing significant disability at hospital discharge (RR 1.00, 95% CI 0.60 to 1.67). There was no significant difference (P = 0.53) in Glasgow Coma Score at discharge between IVIG (median score 14; range 3 to 15) and placebo (median 14 score; range 7 to 15) in the Japanese encephalitis study. The median length of hospital stay in the Japanese encephalitis study was similar for IVIG-treated (median 13 days; range 9 to 21) and placebo-treated (median 12 days; range 6 to 18) children (P = 0.59).Pooled analysis of the results of the other two studies resulted in a significantly lower mean length of hospital stay (MD -4.54 days, 95% CI -7.47 to -1.61; P = 0.002), time to resolution of fever (MD -0.97 days, 95% CI -1.25 to -0.69; P < 0.00001), time to stop spasms (MD -1.49 days, 95% CI -1.97 to -1.01; P < 0.00001), time to regain consciousness (MD -1.10 days, 95% CI -1.48 to -0.72; P < 0.00001), and time to resolution of neuropathic symptoms (MD -3.20 days, 95% CI -3.34 to -3.06; P < 0.00001) in favour of IVIG when compared with standard care.None of the included studies reported other outcomes of interest in this review including need for invasive ventilation, duration of invasive ventilation, cognitive impairment, poor adaptive functioning, quality of life, number of seizures, and new diagnosis of epilepsy.The quality of evidence was very low for all outcomes of this review.
Authors' conclusions: The findings suggest a clinical benefit of adjunctive IVIG treatment for children with viral encephalitis for some clinical measures (i.e. mean length of hospital stay, time (days) to stop spasms, time to regain consciousness, and time to resolution of neuropathic symptoms and fever. For children with Japanese encephalitis, IVIG had a similar effect to placebo when assessing significant disability and serious adverse events.Despite these findings, the risk of bias in the included studies and quality of the evidence make it impossible to reach any firm conclusions on the efficacy and safety of IVIG as add-on treatment for children with encephalitis. Furthermore, the included studies involved only children with viral encephalitis, therefore findings of this review cannot be generalised to all forms of encephalitis. Future well-designed RCTs are needed to assess the efficacy and safety of IVIG in the management of children with all forms of encephalitis. There is a need for internationally agreed core outcome measures for clinical trials in childhood encephalitis.
Conflict of interest statement
MAI: none.
NGM: none.
MA: none.
AJP: none.
Figures
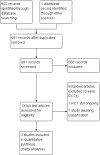
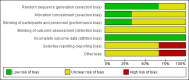






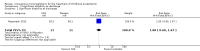









Update of
- doi: 10.1002/14651858.CD011367
References
References to studies included in this review
Chen 2006 {published data only}
-
- Chen D‐M, Li S‐N. The clinical observation of high dose immunoglobulin for children viral encephalitis. Chinese Journal of Primary Medicine and Pharmacy 2006;13(5):816‐7.
Rayamajhi 2015 {published data only}
Wu 2014 {published data only}
-
- Wu H, Wan Z, Liu L, Qiu L, Xiuling D. Comparison of the clinical efficacy of immunoglobulin and interferon in the treatment of hand foot and mouth disease complicated with viral encephalitis. Chinese Journal of Primary Medicine and Pharmacy 2014;21:3127‐8.
References to studies excluded from this review
Bhatt 2012 {published and unpublished data}
-
- Bhatt GC, Sankar J, Kushwaha KP. Use of intravenous immunoglobulin compared with standard therapy is associated with improved clinical outcomes in children with acute encephalitis syndrome complicated by myocarditis. Pediatric Cardiology 2012;33(8):1370‐6. - PubMed
Erol 2013 {published data only}
Gelli 1974 {published data only}
-
- Gelli GP, Arioni G, Laurenti J. Preliminary results of the use of human anti‐measles immunoglobulin in measles encephalitis. Minerva Pediatrica 1974;26(26):1291‐4. - PubMed
Girija 2010 {published data only}
-
- Girija A, RamachandranNair R, Rafeequ M, Manoj P. Multimodal treatment of acute disseminated encephalomyelitis in children. Journal of Pediatric Neurology 2010;8(4):359‐65.
İncecik 2013 {published data only}
-
- İncecik F, Hergüner M, Altunbaşak Ş. Acute disseminated encephalomyelitis: an evaluation of 15 cases in childhood. Turkish Journal of Pediatrics 2013;55(3):253‐9. - PubMed
Růžek 2013 {published data only}
Samileh 2007 {published data only}
-
- Samileh N, Hassan T. Acute disseminated encephalomyelitis in children. A descriptive study in Tehran, Iran. Saudi Medical Journal 2007;28(3):396‐9. - PubMed
Unay 2004 {published data only}
-
- Unay B, Sarici SU, Bulakbaşi N, Akin R, Gökçay E. Intravenous immunoglobulin therapy in acute disseminated encephalomyelitis associated with hepatitis A infection. Pediatrics International 2004;46(2):171‐3. - PubMed
References to studies awaiting assessment
Fan 2010 {published data only}
-
- Fan J‐L. The clinical observation of the high dose intravenous immunoglobulin for severe viral encephalitis. Internal Medicine of China 2010;02:118‐9.
References to ongoing studies
NCT02308982 {unpublished data only}
-
- NCT02308982. Investigating the role of early intravenous immunoglobulin treatment for children with encephalitis [A phase III multi‐centre randomised, double blind, placebo controlled trial to assess the role of intravenous immunoglobulin in the management of children with encephalitis]. clinicaltrials.gov/ct2/show/record/NCT02308982 Date first received; 1 December 2014.
Additional references
Absoud 2013
Aygun 2001
-
- Aygun A, Kabakus N, Celik I, Turgut M, Yoldas T, Gök U, et al. Long term neurological outcome of acute encephalitis. Journal of Tropical Pediatrics 2001;47(4):243‐7. [PUBMED: PMID 111523767] - PubMed
Basta 1996
-
- Basta M, Illa I, Dalakas M C. Increased in vitro uptake of the complement C3b in the serum of patients with Guillain‐Barré syndrome, myasthenia gravis and dermatomyositis. Journal of Neuroimmunology 1996;71:227‐9. - PubMed
Basta 2003
-
- Basta M, Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, et al. F(ab)'2‐mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nature Medicine 2003;9(4):431‐8. - PubMed
Bayley 2006
-
- Bayley N. Bayley Scales of Infant and Toddler Development. Technical Manual. 3rd Edition. San Antonio (TX): Harcourt Assessment, 2006.
Beers 2012
BNF Publications
-
- British National Formulary Publications. British National Formulary for Children (BNF for Children). Chapter 5.3.2: Herpesvirus infections; page 326. London (UK): British Medical Association, the Royal Pharmaceutical Society, the Royal College of Paediatrics and Child Health, the Neonatal and Paediatric Pharmacists Group, 2014‐2015.
Caramello 2006
-
- Caramello P, Canta F, Balbiano R, Lipani F, Ariaudo S, Agostini M, et al. Role of intravenous immunoglobulin administration in Japanese encephalitis. Clinical Infectious Diseases 2006;43(12):1620‐1. [PUBMED: PMID 17109300] - PubMed
Cervantes‐Barragán 2012
-
- Cervantes‐Barragán L, Firner S, Bechmann I, Waisman A, Lahl K, Sparwasser T, et al. Regulatory T cells selectively preserve immune privilege of self‐antigens during viral central nervous system infection. Journal of Immunology (Baltimore, Md. : 1950) 2012;188(8):3678‐85. [PUBMED: PMID: 22407917] - PubMed
Crow 2007
-
- Crow AR, Song S, Semple JW, Freedman J, Lazarus AH. A role for IL‐1 receptor antagonist or other cytokines in the acute therapeutic effects of IVIg?. Blood 2007;109:155‐8. - PubMed
Dalakas 1998
-
- Dalakas M. Mechanism of action of intravenous immunoglobulin and therapeutic considerations in the treatment of autoimmune neurologic diseases. Neurology 1998;51(6 Suppl 5):2‐8. [PUBMED: PMID 9851723] - PubMed
Dalakas 2004
-
- Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence‐based indications and safety profile. Pharmacology & Therapeutics 2004;102:177‐93. - PubMed
Davison 2003
de Aquino 2013
Dietrich 1990
-
- Dietrich G, Kazatchkine MD. Normal immunoglobulin G (IgG) for therapeutic use (intravenous Ig) contain antiidiotypic specificities against an immunodominant, disease‐associated, cross‐reactive idiotype of human anti‐thyroglobulin autoantibodies. Journal of Clinical Investigation 1990;85:620‐5. - PMC - PubMed
Dowell 2000
-
- Dowell E, Easton A, Solomon T. The consequences of encephalitis. Report of a Postal Survey (Year 2000 survey) carried out by the Encephalitis Support Group. www.encephalitis.info/images/iPdf/Research2/CONSEQUENCES1.pdf (accessed 10 April 2014).
Elion 1983
-
- Elion GB. The biochemistry and mechanism of action of acyclovir. Journal of Antimicrobial Chemotherapy 1983;12 Suppl B:9‐17. - PubMed
Elion 1993
-
- Elion GB. Acyclovir: discovery, mechanism of action, and selectivity. Journal of Medical Virology 1993;Suppl 1:2‐6. - PubMed
Esposito 2012
-
- Esposito S, Picciolli I, Semino M, Principi N. Steroids and childhood encephalitis. Pediatric Infectious Disease Journal 2012;31:759‐60. - PubMed
FDA 2013
-
- US Food, Drug Administration. Immune globulin intravenous (IGIV) indications. Page last updated: 6 October 2013. www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/L... (accessed 15 May 2014).
Fowler 2010
-
- Fowler A, Stödberg T, Eriksson M, Wickström R. Long‐term outcomes of acute encephalitis in childhood. Pediatrics 2010;126(4):828‐35. [PUBMED: PMID 20876179] - PubMed
Fowler 2008
-
- Fowler A, Stödberg T, Eriksson M, Wickström R. Childhood encephalitis in Sweden: etiology, clinical presentation and outcome. European Journal of Paediatric Neurology: EJPN 2008;12:484‐90. - PubMed
Frenzel 2012
-
- Frenzel K, Ganepola S, Michel D, Thiel E, Krüger DH, Uharek L, et al. Antiviral function and efficacy of polyvalent immunoglobulin products against CMV isolates in different human cell lines. Medical Microbiology and Immunology 2012;201:277‐86. - PubMed
Gadian 2017
-
- Gadian J, Kirk E, Holliday K, Lim M, Absoud M. Systematic review of immunoglobulin use in paediatric neurological and neurodevelopmental disorders. Developmental Medicine and Child Neurology 2017;59(2):133‐44. - PubMed
Glezer 2003
-
- Glezer I, Munhoz CD, Kawamoto EM, Marcourakis T, Avellar MC, Scavone C. MK‐801 and 7‐Ni attenuate the activation of brain NF‐kappa B induced by LPS. Neuropharmacology 2003;45:1120‐9. - PubMed
GRADEpro 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEprofiler (GRADEpro). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Granerod 2010
-
- Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population‐based prospective study. Lancet Infectious Diseases 2010;10(12):835‐44. [PUBMED: PMID: 20952256] - PubMed
Hacohen 2013
-
- Hacohen Y, Wright S, Waters P, Agrawal S, Carr L, Cross H, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. Journal of Neurology, Neurosurgery, and Psychiatry 2013;84(7):748‐55. [PUBMED: 23175854] - PMC - PubMed
Hacohen 2014
-
- Hacohen Y, Deiva K, Pettingill P, Waters P, Siddiqui A, Chretien P, et al. N‐methyl‐D‐aspartate receptor antibodies in post‐herpes simplex virus encephalitis neurological relapse. Movement Disorders 2014;29:90‐6. - PubMed
Harrison 2003
-
- Harrison PL, Oakland T. ABAS®‐II Adaptive Behavior Assessment System®. 2nd Edition. San Antonio (TX): Harcourt Assessment, 2003.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hughes 2009
ICH 1994
-
- International Conference on Harmonisation. ICH harmonised tripartite guideline clinical safety data management: definitions and standards for expedited reporting E2A. Current step 4 version. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. 27 October 1994. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E... (accessed prior to 25 September 2017).
Iro 2017
-
- Iro MA, Sadarangani M, Goldacre R, Nickless A, Pollard AJ, Goldacre MJ. 30‐year trends in admission rates for encephalitis in children in England and effect of improved diagnostics and measles‐mumps‐rubella vaccination: a population‐based observational study. Lancet Infectious Diseases 2017;17(4):422‐30. - PubMed
Kamei 2005
Kazatchkine 2001
-
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. New England Journal of Medicine 2001;345:747‐55. - PubMed
Kerpel‐Fronius 1983
-
- Kerpel‐Fronius S, Nyerges G, Mészner Z, Eckhardt S, Walker E, Bridgen D. Intravenous acyclovir (ACV, Zovirax) therapy of varicella zoster infections in immunologically endangered patients. Orvosi Hetilap 1983;124(48):2913‐8. - PubMed
Khetsuriani 2002
-
- Khetsuriani N, Holman C, Anderson L. Burden of encephalitis‐associated hospitalizations in the United States, 1988‐1997. Clinical Infectious Diseases 2002;35(2):175‐82. [PUBMED: PMID: 12087524] - PubMed
Kishimoto 2004
-
- Kishimoto C, Hiraoka Y, Takada H. Effects of immunoglobulin upon murine myocarditis caused by influenza A virus: superiority of intact type to F(ab')2 type. Journal of Cardiovascular Pharmacology 2004;43(1):61‐7. - PubMed
Krause 2002
-
- Krause I, Wu R, Sherer Y, Patanik M, Peter J, Shoenfeld Y. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations, a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfusion Medicine (Oxford, England) 2002;12:133‐9. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lewthwaite 2010
Lizarraga 2013
Looney 2006
-
- Looney RJ, Huggins J. Use of intravenous immunoglobulin G (IVIG). Best Practice & Research. Clinical Haematology 2006;19:3‐25. - PubMed
Lundberg 2008
-
- Lundberg P, Ramakrishna C, Brown J, Tyszka J, Hamamura M, Hinton D, et al. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. Journal of Virology 2008;82:7078‐88. [PUBMED: PMCID: PMC2446972; PUBMED: PMID: 18480436] - PMC - PubMed
Lutz 1996
-
- Lutz HU, Stammler P, Jelezarova E, Nater M, Späth PJ. High doses of immunoglobulin G attenuate immune aggregate‐mediated complement activation by enhancing physiologic cleavage of C3b in C3bn‐IgG complexes. Blood 1996;88:184‐93. [PUBMED: PMID: 8704173] - PubMed
Marchioni 2013
-
- Marchioni E, Ravaglia S, Montomoli C, Tavazzi E, Minoli L, Baldanti F, et al. Postinfectious neurologic syndromes: a prospective cohort study. Neurology 2013;80(10):882‐9. [PUBMED: PMID: 23325908] - PubMed
Martinez‐Torres 2008
-
- Martinez‐Torres F, Menon S, Pritsch M, Victor N, Jenetzky E, Jensen K, et al. Protocol for German trial of Acyclovir and Corticosteroids in Herpes‐simplex‐virus‐Encephalitis (GACHE): a multicenter, multinational, randomized, double‐blind, placebo‐controlled German, Austrian and Dutch trial [ISRCTN45122933]. BMC Neurology 2008;8:40. - PMC - PubMed
Matza 2004
-
- Matza LS, Swensen AR, Flood EM, Secnik K, Leidy NK. Assessment of health‐related quality of life in children: a review of conceptual, methodological, and regulatory issues. Value in Health 2004;7:79‐92. - PubMed
McKay 1999
-
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor‐kappa B and steroid receptor‐signaling pathways. Endocrine Reviews 1999;20(4):435‐59. - PubMed
Meyding‐Lamadé 2002
-
- Meyding‐Lamadé U, Seyfer S, Haas J, Dvorak F, Kehm R, Lamadé W, et al. Experimental herpes simplex virus encephalitis: inhibition of the expression of inducible nitric oxide synthase in mouse brain tissue. Neuroscience Letters 2002;318:21‐4. - PubMed
Mollnes 1995
-
- Mollnes TE, Høgåsen K, Hoaas BF, Michaelsen TE, Garred P, Harboe M. Inhibition of complement‐mediated red cell lysis by immunoglobulins is dependent on the IG isotype and its C1 binding properties. Scandinavian Journal of Immunology 1995;41:449‐56. [PUBMED: PMID:7725063] - PubMed
Ramakrishna 2013
Ramos‐Estebanez 2014
-
- Ramos‐Estebanez C, Lizarraga KJ, Merenda A. A systematic review on the role of adjunctive corticosteroids in herpes simplex virus encephalitis: is timing critical for safety and efficacy?. Antiviral Therapy 2014;19:133‐9. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynolds 2004
-
- Reynolds CR, Kamphaus RW. Assessment of effective intervention. Behavior Assessment System for Children (BASC‐2). 2nd Edition. Vol. 32, Crowley (TX): AGS Publishing, 2004:121‐4.
Rossi 1988
Rozenberg 2013
-
- Rozenberg F. Acute viral encephalitis. Handbook of Clinical Neurology / Edited by P.J. Vinken and G.W. Bruyn 2013;112:1171‐81. - PubMed
Sellner 2005
-
- Sellner J, Dvorak F, Zhou Y, Haas J, Kehm R, Wildemann B, et al. Acute and long‐term alteration of chemokine mRNA expression after anti‐viral and anti‐inflammatory treatment in herpes simplex virus encephalitis. Neuroscience Letters 2005;374:197‐202. - PubMed
Sergerie 2007
-
- Sergerie Y, Boivin G, Gosselin D, Rivest S. Delayed but not early glucocorticoid treatment protects the host during experimental herpes simplex virus encephalitis in mice. Journal of Infectious Diseases 2007;195:817‐25. - PubMed
Sparrow 1986
-
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales. American Guidance Service, Circle Pines. Journal of Educational Measurement 1986;23(4):389‐91.
Sparrow 1993
-
- Sparrow SS, Carter AS, Cicchetti DV. Vineland Screener: Overview, Reliability, Validity, Administration and Scoring. Interview Edition, Survey Form Manual. New Haven (CT): Yale University Child Study Centre, 1993.
Stangel 1997
-
- Stangel M, Schumacher HC, Ruprecht K, Boegner F, Marx P. Immunoglobulins for intravenous use inhibit TNF alpha cytotoxicity in vitro. Immunological Investigations 1997;26:569‐78. - PubMed
Tha‐In 2008
-
- Tha‐In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J. Modulation of the cellular immune system by intravenous immunoglobulin. Trends in Immunology 2008;29(12):608‐15. [PUBMED: PMID: 18926775] - PubMed
Thompson 2000
-
- Thompson KA, Blessing W, Wesselingh SL. Herpes simplex replication and dissemination is not increased by corticosteroid treatment in a rat model of focal herpes encephalitis. Journal of Neurovirology 2000;6:25‐32. - PubMed
Thompson 2012
-
- Thompson C, Kneen R, Riordan A, Kelly D, Pollard A J. Encephalitis in children. Archives of Disease in Childhood 2012;97:150‐61. [PUBMED: PMID: 21715390] - PubMed
Titulaer 2013
Trinath 2013
-
- Trinath J, Hegde P, Sharma M, Maddur MS, Rabin M, Vallat JM, et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase‐2‐dependent prostaglandin E2 in human dendritic cells. Blood 2013;122(8):1419‐27. [PUBMED: PMID: 23847198] - PubMed
UK Trialists
-
- UK Trialists. Individual patient data (as supplied 30 April 2013). Data on File.
van Swieten 1988
-
- Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; Vol. 19, issue 5:604‐7. - PubMed
Varni 1999
-
- Varni JW, Seid M, Rode CA. The PedsQL™: measurement model for the pediatric quality of life inventory. Medical Care 1999; Vol. 37, issue 2:126‐39. - PubMed
Venkatesan 2013
-
- Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clinical Infectious Diseases 2013;57(8):1114‐28. [PUBMED: PMID 23861361] - PMC - PubMed
Vora 2014
-
- Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. Burden of encephalitis‐associated hospitalizations in the United States, 1998‐2010. Neurology 2014;82(5):443‐51. [PUBMED: PMID: 24384647] - PubMed
Wang 2006
-
- Wang SM, Lei HY, Huang MC, Su LY, Lin HC, Yu CK, et al. Modulation of cytokine production by intravenous immunoglobulin in patients with enterovirus 71‐associated brainstem encephalitis. Journal of Clinical Virology 2006;37(1):47‐52. [PUBMED: PMID: 16861032] - PubMed
Wechsler 2002
-
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence (WPPSI ‐ III). 3rd Edition. Ontario (Canada): Pearson Canada Assessment Inc, 2002.
Wechsler 2003
-
- Wechsler D. Wechsler Intelligence Scale for Children (WISC‐IV). 4th Edition. Ontario (Canada): Pearson Canada Assessment Inc, 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

