Structural Basis for G Protein-Coupled Receptor Activation
- PMID: 28967738
- PMCID: PMC6613644
- DOI: 10.1021/acs.biochem.7b00747
Structural Basis for G Protein-Coupled Receptor Activation
Abstract
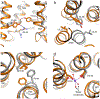
G protein-coupled receptors (GPCRs) are critical regulators of human physiology and make up the largest single class of therapeutic drug targets. Although GPCRs regulate highly diverse physiology, they share a common signaling mechanism whereby extracellular stimuli induce conformational changes in the receptor that enable activation of heterotrimeric G proteins and other intracellular effectors. Advances in GPCR structural biology have made it possible to examine ligand-induced GPCR activation at an unprecedented level of detail. Here, we review the structural basis for family A GPCR activation, with a focus on GPCRs for which structures are available in both active or active-like states and inactive states. Crystallographic and other biophysical data show how chemically diverse ligands stabilize highly conserved conformational changes on the intracellular side of the receptors, allowing many different extracellular stimuli to utilize shared downstream signaling molecules. Finally, we discuss the remaining challenges in understanding GPCR activation and signaling and highlight new technologies that may allow unanswered questions to be resolved.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures

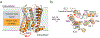

References
-
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, and Kobilka BK (2007) GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 318, 1266–1273. - PubMed
-
- Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Chandra R, Goren MA, Kruse AC, Nurva S, Loland CJ, Pierre Y, Drew D, Popot JL, Picot D, Fox BG, Guan L, Gether U, Byrne B, Kobilka B, and Gellman SH (2010) Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat. Methods 7, 1003–1008. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

