Using a novel computational drug-repositioning approach (DrugPredict) to rapidly identify potent drug candidates for cancer treatment
- PMID: 28967908
- PMCID: PMC5799769
- DOI: 10.1038/onc.2017.328
Using a novel computational drug-repositioning approach (DrugPredict) to rapidly identify potent drug candidates for cancer treatment
Abstract
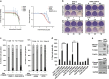
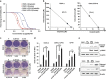
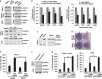
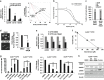
Computation-based drug-repurposing/repositioning approaches can greatly speed up the traditional drug discovery process. To date, systematic and comprehensive computation-based approaches to identify and validate drug-repositioning candidates for epithelial ovarian cancer (EOC) have not been undertaken. Here, we present a novel drug discovery strategy that combines a computational drug-repositioning system (DrugPredict) with biological testing in cell lines in order to rapidly identify novel drug candidates for EOC. DrugPredict exploited unique repositioning opportunities rendered by a vast amount of disease genomics, phenomics, drug treatment, and genetic pathway and uniquely revealed that non-steroidal anti-inflammatories (NSAIDs) rank just as high as currently used ovarian cancer drugs. As epidemiological studies have reported decreased incidence of ovarian cancer associated with regular intake of NSAIDs, we assessed whether NSAIDs could have chemoadjuvant applications in EOC and found that (i) NSAID Indomethacin induces robust cell death in primary patient-derived platinum-sensitive and platinum- resistant ovarian cancer cells and ovarian cancer stem cells and (ii) downregulation of β-catenin is partially driving effects of Indomethacin in cisplatin-resistant cells. In summary, we demonstrate that DrugPredict represents an innovative computational drug- discovery strategy to uncover drugs that are routinely used for other indications that could be effective in treating various cancers, thus introducing a potentially rapid and cost-effective translational opportunity. As NSAIDs are already in routine use in gynecological treatment regimens and have acceptable safety profile, our results will provide with a rationale for testing NSAIDs as potential chemoadjuvants in EOC patient trials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Critical role of Wnt/β-catenin signaling in driving epithelial ovarian cancer platinum resistance.Oncotarget. 2015 Sep 15;6(27):23720-34. doi: 10.18632/oncotarget.4690. Oncotarget. 2015. PMID: 26125441 Free PMC article.
-
Elevated β-catenin activity contributes to carboplatin resistance in A2780cp ovarian cancer cells.Biochem Biophys Res Commun. 2015 Dec 4-11;468(1-2):173-8. doi: 10.1016/j.bbrc.2015.10.138. Epub 2015 Oct 29. Biochem Biophys Res Commun. 2015. PMID: 26522223
-
Downregulation of RIF1 Enhances Sensitivity to Platinum-Based Chemotherapy in Epithelial Ovarian Cancer (EOC) by Regulating Nucleotide Excision Repair (NER) Pathway.Cell Physiol Biochem. 2018;46(5):1971-1984. doi: 10.1159/000489418. Epub 2018 Apr 26. Cell Physiol Biochem. 2018. PMID: 29719287
-
Current state and outlook for drug repositioning anticipated in the field of ovarian cancer.J Gynecol Oncol. 2019 Jan;30(1):e10. doi: 10.3802/jgo.2019.30.e10. Epub 2018 Oct 10. J Gynecol Oncol. 2019. PMID: 30479094 Free PMC article. Review.
-
Efficacy of trebananib (AMG 386) in treating epithelial ovarian cancer.Expert Opin Pharmacother. 2016;17(6):853-60. doi: 10.1517/14656566.2016.1161027. Epub 2016 Mar 21. Expert Opin Pharmacother. 2016. PMID: 26933765 Review.
Cited by
-
Predicting associations among drugs, targets and diseases by tensor decomposition for drug repositioning.BMC Bioinformatics. 2019 Dec 16;20(Suppl 26):628. doi: 10.1186/s12859-019-3283-6. BMC Bioinformatics. 2019. PMID: 31839008 Free PMC article.
-
A Proteotranscriptomic-Based Computational Drug-Repositioning Method for Alzheimer's Disease.Front Pharmacol. 2020 Jan 29;10:1653. doi: 10.3389/fphar.2019.01653. eCollection 2019. Front Pharmacol. 2020. PMID: 32063857 Free PMC article.
-
Overcoming cancer therapeutic bottleneck by drug repurposing.Signal Transduct Target Ther. 2020 Jul 2;5(1):113. doi: 10.1038/s41392-020-00213-8. Signal Transduct Target Ther. 2020. PMID: 32616710 Free PMC article. Review.
-
Exploring the new horizons of drug repurposing: A vital tool for turning hard work into smart work.Eur J Med Chem. 2019 Nov 15;182:111602. doi: 10.1016/j.ejmech.2019.111602. Epub 2019 Aug 8. Eur J Med Chem. 2019. PMID: 31421629 Free PMC article. Review.
-
Gut-microbiota-microglia-brain interactions in Alzheimer's disease: knowledge-based, multi-dimensional characterization.Alzheimers Res Ther. 2021 Oct 20;13(1):177. doi: 10.1186/s13195-021-00917-1. Alzheimers Res Ther. 2021. PMID: 34670619 Free PMC article.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. - PubMed
-
- Cooke SL, Brenton JD. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol 2011; 12: 1169–1174. - PubMed
-
- Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov 2011; 10: 507–519. - PubMed
-
- Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 2012; 11: 191–200. - PubMed
-
- Hurle MR, Yang L, Xie Q, Rajpal DK, Sanseau P, Agarwal P. Computational drug repositioning: from data to therapeutics. Clin Pharmacol Ther 2013; 93: 335–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

