Mechanical confinement regulates cartilage matrix formation by chondrocytes
- PMID: 28967913
- PMCID: PMC5701824
- DOI: 10.1038/nmat4993
Mechanical confinement regulates cartilage matrix formation by chondrocytes
Abstract
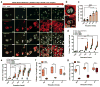
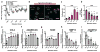
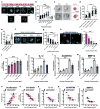
Cartilage tissue equivalents formed from hydrogels containing chondrocytes could provide a solution for replacing damaged cartilage. Previous approaches have often utilized elastic hydrogels. However, elastic stresses may restrict cartilage matrix formation and alter the chondrocyte phenotype. Here we investigated the use of viscoelastic hydrogels, in which stresses are relaxed over time and which exhibit creep, for three-dimensional (3D) culture of chondrocytes. We found that faster relaxation promoted a striking increase in the volume of interconnected cartilage matrix formed by chondrocytes. In slower relaxing gels, restriction of cell volume expansion by elastic stresses led to increased secretion of IL-1β, which in turn drove strong up-regulation of genes associated with cartilage degradation and cell death. As no cell-adhesion ligands are presented by the hydrogels, these results reveal cell sensing of cell volume confinement as an adhesion-independent mechanism of mechanotransduction in 3D culture, and highlight stress relaxation as a key design parameter for cartilage tissue engineering.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

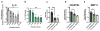
Similar articles
-
Hydrazone covalent adaptable networks modulate extracellular matrix deposition for cartilage tissue engineering.Acta Biomater. 2019 Jan 1;83:71-82. doi: 10.1016/j.actbio.2018.11.014. Epub 2018 Nov 10. Acta Biomater. 2019. PMID: 30419278 Free PMC article.
-
In vitro expression of cartilage-specific markers by chondrocytes on a biocompatible hydrogel: implications for engineering cartilage tissue.Cell Transplant. 2001;10(8):755-63. Cell Transplant. 2001. PMID: 11814119
-
Interconnected macroporous poly(ethylene glycol) cryogels as a cell scaffold for cartilage tissue engineering.Tissue Eng Part A. 2010 Oct;16(10):3033-41. doi: 10.1089/ten.TEA.2010.0045. Tissue Eng Part A. 2010. PMID: 20486791
-
Matrix Viscoelasticity Tunes the Mechanobiological Behavior of Chondrocytes.Cell Biochem Funct. 2024 Sep;42(7):e4126. doi: 10.1002/cbf.4126. Cell Biochem Funct. 2024. PMID: 39324844 Review.
-
Effects of shear stress on articular chondrocyte metabolism.Biorheology. 2000;37(1-2):95-107. Biorheology. 2000. PMID: 10912182 Review.
Cited by
-
3D bioprinting of in situ vascularized tissue engineered bone for repairing large segmental bone defects.Mater Today Bio. 2022 Aug 8;16:100382. doi: 10.1016/j.mtbio.2022.100382. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36033373 Free PMC article.
-
Progress in mimicking brain microenvironments to understand and treat neurological disorders.APL Bioeng. 2021 Apr 8;5(2):020902. doi: 10.1063/5.0043338. eCollection 2021 Jun. APL Bioeng. 2021. PMID: 33869984 Free PMC article.
-
Biomimetic hydrogel blanket for conserving and recovering intrinsic cell properties.Biomater Res. 2022 Dec 13;26(1):78. doi: 10.1186/s40824-022-00327-w. Biomater Res. 2022. PMID: 36514131 Free PMC article.
-
Snake venom-defined fibrin architecture dictates fibroblast survival and differentiation.Nat Commun. 2023 Feb 23;14(1):1029. doi: 10.1038/s41467-023-36437-9. Nat Commun. 2023. PMID: 36823141 Free PMC article.
-
Tunable Mesoscopic Collagen Island Architectures Modulate Stem Cell Behavior.Adv Mater. 2023 Apr;35(16):e2207882. doi: 10.1002/adma.202207882. Epub 2023 Mar 9. Adv Mater. 2023. PMID: 36895051 Free PMC article.
References
-
- Getgood A, Brooks R, Fortier L, Rushton N. Articular cartilage tissue engineering: today’s research, tomorrow’s practice? J Bone Joint Surg Br. 2009;91:565–576. - PubMed
-
- Guettler JH. Osteochondral Defects in the Human Knee: Influence of Defect Size on Cartilage Rim Stress and Load Redistribution to Surrounding Cartilage. Am J Sports Med. 2004;32:1451–1458. - PubMed
-
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. J Med. 1994;331:889–895. - PubMed
-
- Gibson AJ, McDonnell SM, Price AJ. Matrix-Induced Autologous Chondrocyte Implantation. Oper Tech Orthop. 2006;16:262–265.
-
- Steadman JR, Rodkey WG, Briggs KK, Rodrigo JJ. The microfracture technic in the management of complete cartilage defects in the knee joint. Orthopade. 1999;28:26–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

