C-Terminal Truncated α-Synuclein Fibrils Contain Strongly Twisted β-Sheets
- PMID: 28968082
- PMCID: PMC5668890
- DOI: 10.1021/jacs.7b07403
C-Terminal Truncated α-Synuclein Fibrils Contain Strongly Twisted β-Sheets
Abstract
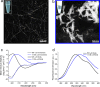
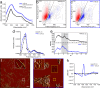
C-terminal truncations of monomeric wild-type alpha-synuclein (henceforth WT-αS) have been shown to enhance the formation of amyloid aggregates both in vivo and in vitro and have been associated with accelerated progression of Parkinson's disease (PD). The correlation with PD may not solely be a result of faster aggregation, but also of which fibril polymorphs are preferentially formed when the C-terminal residues are deleted. Considering that different polymorphs are known to result in distinct pathologies, it is important to understand how these truncations affect the organization of αS into fibrils. Here we present high-resolution microscopy and advanced vibrational spectroscopy studies that indicate that the C-terminal truncation variant of αS, lacking residues 109-140 (henceforth referred to as 1-108-αS), forms amyloid fibrils with a distinct structure and morphology. The 1-108-αS fibrils have a unique negative circular dichroism band at ∼230 nm, a feature that differs from the canonical ∼218 nm band usually observed for amyloid fibrils. We show evidence that 1-108-αS fibrils consist of strongly twisted β-sheets with an increased inter-β-sheet distance and a higher solvent exposure than WT-αS fibrils, which is also indicated by the pronounced differences in the 1D-IR (FTIR), 2D-IR, and vibrational circular dichroism spectra. As a result of their distinct β-sheet structure, 1-108-αS fibrils resist incorporation of WT-αS monomers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Evidence for Intramolecular Antiparallel Beta-Sheet Structure in Alpha-Synuclein Fibrils from a Combination of Two-Dimensional Infrared Spectroscopy and Atomic Force Microscopy.Sci Rep. 2017 Jan 23;7:41051. doi: 10.1038/srep41051. Sci Rep. 2017. PMID: 28112214 Free PMC article.
-
The effect of truncation on prion-like properties of α-synuclein.J Biol Chem. 2018 Sep 7;293(36):13910-13920. doi: 10.1074/jbc.RA118.001862. Epub 2018 Jul 20. J Biol Chem. 2018. PMID: 30030380 Free PMC article.
-
NMR unveils an N-terminal interaction interface on acetylated-α-synuclein monomers for recruitment to fibrils.Proc Natl Acad Sci U S A. 2021 May 4;118(18):e2017452118. doi: 10.1073/pnas.2017452118. Proc Natl Acad Sci U S A. 2021. PMID: 33903234 Free PMC article.
-
Protein denaturation and aggregation: Cellular responses to denatured and aggregated proteins.Ann N Y Acad Sci. 2005 Dec;1066:181-221. doi: 10.1196/annals.1363.030. Ann N Y Acad Sci. 2005. PMID: 16533927 Review.
-
Disruptive membrane interactions of alpha-synuclein aggregates.Biochim Biophys Acta Proteins Proteom. 2019 May;1867(5):468-482. doi: 10.1016/j.bbapap.2018.10.006. Epub 2018 Oct 11. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 30315896 Review.
Cited by
-
Carboxy-terminal truncation and phosphorylation of α-synuclein elongates survival in a prion-like seeding mouse model of synucleinopathy.Neurosci Lett. 2020 Jul 27;732:135017. doi: 10.1016/j.neulet.2020.135017. Epub 2020 May 1. Neurosci Lett. 2020. PMID: 32371157 Free PMC article.
-
Cellular processing of α-synuclein fibrils results in distinct physiological C-terminal truncations with a major cleavage site at residue Glu 114.J Biol Chem. 2023 Jul;299(7):104912. doi: 10.1016/j.jbc.2023.104912. Epub 2023 Jun 10. J Biol Chem. 2023. PMID: 37307916 Free PMC article.
-
Carboxy-terminal truncations of mouse α-synuclein alter aggregation and prion-like seeding.FEBS Lett. 2020 Apr;594(8):1271-1283. doi: 10.1002/1873-3468.13728. Epub 2020 Jan 24. FEBS Lett. 2020. PMID: 31912891 Free PMC article.
-
Modulation of supramolecular self-assembly of an antimicrobial designer peptide by single amino acid substitution: Implications on peptide activity.Nanoscale Adv. 2019 Dec 1;1(12):4679-4682. doi: 10.1039/c9na00498j. Epub 2019 Oct 31. Nanoscale Adv. 2019. PMID: 31844837 Free PMC article.
-
Molecular Dynamics Study of α-Synuclein Domain Deletion Mutant Monomers.bioRxiv [Preprint]. 2025 Jan 20:2024.03.23.586267. doi: 10.1101/2024.03.23.586267. bioRxiv. 2025. PMID: 38586052 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

