Breast cancer cell-derived fibroblast growth factors enhance osteoclast activity and contribute to the formation of metastatic lesions
- PMID: 28968431
- PMCID: PMC5624603
- DOI: 10.1371/journal.pone.0185736
Breast cancer cell-derived fibroblast growth factors enhance osteoclast activity and contribute to the formation of metastatic lesions
Abstract
Fibroblast growth factors (FGFs) and their receptors (FGFRs) have been implicated in promoting breast cancer growth and progression. While the autocrine effects of FGFR activation in tumor cells have been extensively studied, little is known about the effects of tumor cell-derived FGFs on cells in the microenvironment. Because FGF signaling has been implicated in the regulation of bone formation and osteoclast differentiation, we hypothesized that tumor cell-derived FGFs are capable of modulating osteoclast function and contributing to growth of metastatic lesions in the bone. Initial studies examining FGFR expression during osteoclast differentiation revealed increased expression of FGFR1 in osteoclasts during differentiation. Therefore, studies were performed to determine whether tumor cell-derived FGFs are capable of promoting osteoclast differentiation and activity. Using both non-transformed and transformed cell lines, we demonstrate that breast cancer cells express a number of FGF ligands that are known to activate FGFR1. Furthermore our results demonstrate that inhibition of FGFR activity using the clinically relevant inhibitor BGJ398 leads to reduced osteoclast differentiation and activity in vitro. Treatment of mice injected with tumor cells into the femurs with BGJ398 leads to reduced osteoclast activity and bone destruction. Together, these studies demonstrate that tumor cell-derived FGFs enhance osteoclast function and contribute to the formation of metastatic lesions in breast cancer.
Conflict of interest statement
Figures

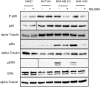



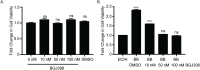

References
-
- ACS. Breast Cancer Facts and Figures. American Cancer Society; 2014.
-
- Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. Epub 2005/08/02. doi: 10.1038/nrc1670 . - DOI - PubMed
-
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802. Epub 2005/08/27. doi: 10.1056/NEJMoa050434 . - DOI - PubMed
-
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–7. doi: 10.1200/JCO.2009.25.9820 . - DOI - PubMed
-
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–25. Epub 2011/05/20. doi: 10.1038/nrc3055 . - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

