Modulators of 14-3-3 Protein-Protein Interactions
- PMID: 28968506
- PMCID: PMC5949722
- DOI: 10.1021/acs.jmedchem.7b00574
Modulators of 14-3-3 Protein-Protein Interactions
Abstract
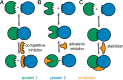
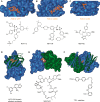
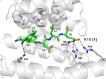
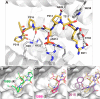
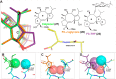
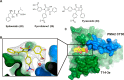
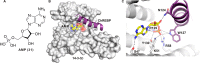
Direct interactions between proteins are essential for the regulation of their functions in biological pathways. Targeting the complex network of protein-protein interactions (PPIs) has now been widely recognized as an attractive means to therapeutically intervene in disease states. Even though this is a challenging endeavor and PPIs have long been regarded as "undruggable" targets, the last two decades have seen an increasing number of successful examples of PPI modulators, resulting in growing interest in this field. PPI modulation requires novel approaches and the integrated efforts of multiple disciplines to be a fruitful strategy. This perspective focuses on the hub-protein 14-3-3, which has several hundred identified protein interaction partners, and is therefore involved in a wide range of cellular processes and diseases. Here, we aim to provide an integrated overview of the approaches explored for the modulation of 14-3-3 PPIs and review the examples resulting from these efforts in both inhibiting and stabilizing specific 14-3-3 protein complexes by small molecules, peptide mimetics, and natural products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




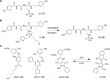


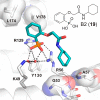
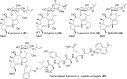

References
-
- Hatzivassiliou G.; Song K.; Yen I.; Brandhuber B. J.; Anderson D. J.; Alvarado R.; Ludlam M. J.; Stokoe D.; Gloor S. L.; Vigers G.; Morales T.; Aliagas I.; Liu B.; Sideris S.; Hoeflich K. P.; Jaiswal B. S.; Seshagiri S.; Koeppen H.; Belvin M.; Friedman L. S.; Malek S. RAF Inhibitors Prime Wild-Type RAF to Activate the MAPK Pathway and Enhance Growth. Nature 2010, 464 (7287), 431–435. 10.1038/nature08833. - DOI - PubMed
-
- Poulikakos P. I.; Persaud Y.; Janakiraman M.; Kong X.; Ng C.; Moriceau G.; Shi H.; Atefi M.; Titz B.; Gabay M. T.; Salton M.; Dahlman K. B.; Tadi M.; Wargo J. A.; Flaherty K. T.; Kelley M. C.; Misteli T.; Chapman P. B.; Sosman J. A.; Graeber T. G.; Ribas A.; Lo R. S.; Rosen N.; Solit D. B. RAF Inhibitor Resistance Is Mediated by Dimerization of Aberrantly Spliced BRAF(V600E). Nature 2011, 480 (7377), 387–390. 10.1038/nature10662. - DOI - PMC - PubMed
-
- Heidorn S. J.; Milagre C.; Whittaker S.; Nourry A.; Niculescu-Duvas I.; Dhomen N.; Hussain J.; Reis-Filho J. S.; Springer C. J.; Pritchard C.; Marais R. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell 2010, 140 (2), 209–221. 10.1016/j.cell.2009.12.040. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

