Evaluating Treatment Efficacy in a Mouse Model of Enterovirus D68-Associated Paralytic Myelitis
- PMID: 28968718
- PMCID: PMC5853295
- DOI: 10.1093/infdis/jix468
Evaluating Treatment Efficacy in a Mouse Model of Enterovirus D68-Associated Paralytic Myelitis
Abstract
Background: Enterovirus D68 (EV-D68)-associated acute flaccid myelitis (AFM) is a devastating neurological disease for which there are no treatments of proven efficacy. The unpredictable temporal and geographic distribution of cases and the rarity of the disease make it unlikely that data from randomized controlled trials will be available to guide therapeutic decisions. We evaluated the following 3 widely used empirical therapies for the ability to reduce the severity of paralysis in a mouse model of EV-D68 infection: (1) human intravenous immunoglobulin (hIVIG), (2) fluoxetine, and (3) dexamethasone.
Methods: Neonatal mice were injected intramuscularly with a human 2014 EV-D68 isolate that reliably induces paralysis in mice due to infection and loss of spinal cord motor neurons. Mice receiving treatments were evaluated for motor impairment, mortality, and spinal cord viral load.
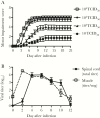
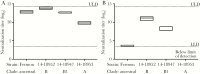
Results: hIVIG, which contained neutralizing antibodies to EV-D68, reduced paralysis in infected mice and decreased spinal cord viral loads. Fluoxetine had no effect on motor impairment or viral loads. Dexamethasone treatment worsened motor impairment, increased mortality, and increased viral loads.
Conclusion: Results in this model of EV-D68-associated AFM provide a rational basis for selecting empirical therapy in humans and establish this model as a useful system for evaluating other potential therapies.
Keywords: Enterovirus D68; acute flaccid myelitis; mouse model; paralysis; therapies.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures



References
-
- Oberste MS, Maher K, Schnurr D et al. . Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–84. - PubMed
-
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA; Centers for Disease Control and Prevention Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 2006; 55:1–20. - PubMed
-
- Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol 1967; 85:297–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

