Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study
- PMID: 28968735
- PMCID: PMC5850284
- DOI: 10.1093/rheumatology/kex301
Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study
Abstract
Objectives: To assess long-term efficacy, safety and tolerability of secukinumab up to 104 weeks in patients with active PsA.
Methods: Patients with PsA (n = 397) were randomized to s.c. secukinumab 300, 150 or 75 mg or placebo at baseline, weeks 1, 2, 3 and 4 and every 4 weeks thereafter. Placebo-treated patients were re-randomized to receive secukinumab 300 or 150 mg s.c. from week 16 (placebo non-responders) or week 24 (placebo responders). Exploratory endpoints at week 104 included 20, 50 and 70% improvement in ACR criteria (ACR20, 50, 70); 75 and 90% improvement in the Psoriasis Area Severity Index, 28-joint DAS with CRP, presence of dactylitis and enthesitis and other patient-reported outcomes. For binary variables, missing values were imputed; continuous variables were analysed by a mixed-effects model for repeated measures.
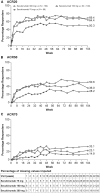
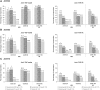
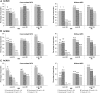
Results: A total of 86/100 (86%), 76/100 (76%) and 65/99 (66%) patients in the secukinumab 300, 150 and 75 mg groups, respectively, completed 104 weeks. At week 104, ACR20 response rates after multiple imputation in the 300, 150 and 75 mg groups were 69.4, 64.4 and 50.3%, respectively. Sustained clinical improvements were observed through week 104 with secukinumab across other clinically important domains of PsA. Responses were sustained through week 104 regardless of prior anti-TNF-α use. Over the entire treatment period the incidence, type and severity of adverse events were consistent with those reported previously.
Conclusion: Secukinumab provided sustained improvements in signs and symptoms and multiple clinical domains in patients of active PsA through 2 years of therapy. Secukinumab was well tolerated, with a safety profile consistent with that reported previously.
Trial registration: ClinicalTrials.gov (https://clinicaltrials.gov), NCT01752634.
Keywords: anti-TNF therapy; biological therapies; efficacy; interleukin; joints; long term; psoriatic arthritis; safety; spondyloarthritis; swelling.
© The Author 2017. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol 2003;4:441–7. - PubMed
-
- Boehncke WH, Menter A.. Burden of disease: psoriasis and psoriatic arthritis. Am J Clin Dermatol 2013;14:377–88. - PubMed
-
- Langley RG, Elewski BE, Lebwohl M. et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med 2014;371:326–38. - PubMed
-
- Ash Z, Gaujoux-Viala C, Gossec L. et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

