A Link Between a Common Mutation in CFTR and Impaired Innate and Adaptive Viral Defense
- PMID: 28968805
- PMCID: PMC5853514
- DOI: 10.1093/infdis/jix474
A Link Between a Common Mutation in CFTR and Impaired Innate and Adaptive Viral Defense
Abstract
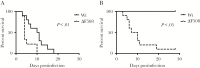
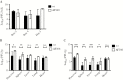
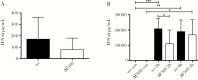
Acute respiratory virus infections predispose the cystic fibrosis (CF) lung to chronic bacterial colonization, which contributes to high mortality. For reasons unknown, respiratory virus infections have a prolonged duration in CF. Here, we demonstrate that mice carrying the most frequent cystic fibrosis transmembrane conductance regulator (CFTR) mutation in humans, ΔF508, show increased morbidity and mortality following infection with a common human enterovirus. ΔF508 mice demonstrated impaired viral clearance, a slower type I interferon response and delayed production of virus-neutralizing antibodies. While the ΔF508 mice had a normal immune cell repertoire, unchanged serum immunoglobulin concentrations and an intact immune response to a T-cell-independent antigen, their response to a T-cell-dependent antigen was significantly delayed. Our studies reveal a novel function for CFTR in antiviral immunity and demonstrate that the ΔF508 mutation in cftr is coupled to an impaired adaptive immune response. This important insight could open up new approaches for patient care and treatment.
Keywords: Coxsackievirus; antibody response; antiviral defense; cystic fibrosis; immunity.
© The Author(s) 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
-
- Elborn JS. Cystic fibrosis. Lancet 2016; 388:2519–31. - PubMed
-
- Billard L, Le Berre R, Pilorge L, Payan C, Hery-Arnaud G, Vallet S. Viruses in cystic fibrosis patients’ airways. Crit Rev Microbiol 2017; 43:1–19. - PubMed
-
- Flight W, Jones A. The diagnosis and management of respiratory viral infections in cystic fibrosis. Expert Rev Respir Med 2017; 11:221–7. - PubMed
-
- Dijkema JS, van Ewijk BE, Wilbrink B, Wolfs TF, Kimpen JL, van der Ent CK. Frequency and duration of rhinovirus infections in children with cystic fibrosis and healthy controls: a longitudinal cohort study. Pediatr Infect Dis J 2016; 35:379–83. - PubMed
-
- van Ewijk BE, van der Zalm MM, Wolfs TF et al. . Prevalence and impact of respiratory viral infections in young children with cystic fibrosis: prospective cohort study. Pediatrics 2008; 122:1171–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

