BMI-1 is a potential therapeutic target in diffuse intrinsic pontine glioma
- PMID: 28968963
- PMCID: PMC5609895
- DOI: 10.18632/oncotarget.18002
BMI-1 is a potential therapeutic target in diffuse intrinsic pontine glioma
Abstract
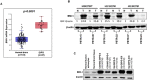
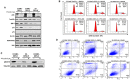
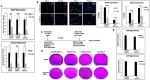
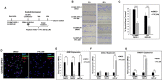
Diffuse intrinsic pontine glioma (DIPG) is a poor-prognosis pediatric brain tumor. No effective curative therapy is currently available and no therapeutic advances have been made in several decades. BMI-1 is a member of the multimeric protein complex Polycomb repressor complex 1. It is highly expressed in a number of diseases and malignancies and has been implicated in self-renewal of normal and cancer cells, and in DNA damage signaling. The role of BMI-1 in DIPG is largely unknown. Here, we show that BMI-1 is highly expressed in tumor tissue samples of DIPG patients and in patient-derived cancer stem-like cells. BMI-1 downregulation leads to the inhibition of DIPG patient-derived neurosphere cell proliferation, cell cycle signaling, self-renewal, telomerase expression and activity, and suppresses DIPG cell migration. Moreover, targeted inhibition of BMI-1 sensitizes DIPG cells to radiomimetic drug-induced DNA damage. Together, our data validate BMI-1 as a potential therapeutic target to treat children with DIPG.
Keywords: BMI-1; DIPG; cancer stem cells; cell proliferation; therapeutic target.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflict of interest.
Figures




References
-
- Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il'yasova D, Kruchko C, McCarthy BJ, Rajaraman P, Schwartzbaum JA, Sadetzki S, Schlehofer B, Tihan T, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113:1953–68. doi: 10.1002/cncr.23741. - DOI - PMC - PubMed
-
- Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tönjes M, Hovestadt V, Albrecht S, Kool M, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–31. doi: 10.1038/nature10833. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

