Isothiocyanates suppress the invasion and metastasis of tumors by targeting FAK/MMP-9 activity
- PMID: 28969043
- PMCID: PMC5609975
- DOI: 10.18632/oncotarget.19213
Isothiocyanates suppress the invasion and metastasis of tumors by targeting FAK/MMP-9 activity
Abstract
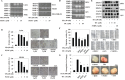
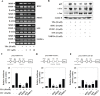
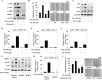
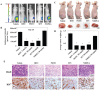
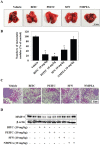
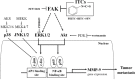
Isothiocyanates, which are present as glucosinolate precursors in cruciferous vegetables, have strong activity against various cancers. Here, we compared the anti-metastatic effects of isothiocyanates (benzyl isothiocyanate (BITC), phenethyl isothiocyanate (PEITC), and sulforaphane (SFN)) by examining how they regulate MMP-9 expression. Isothiocyanates, particularly PEITC, suppressed 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced MMP-9 activity and invasion in various cancer cell lines. By contrast, N-methyl phenethylamine, a PEITC analog without an isothiocyanate functional group, had no effect. A reporter gene assay demonstrated that BITC, PEITC, and SFN suppressed TAP-induced MMP-9 expression by inhibiting AP-1 and NF-κB in U20S osteosarcoma cells. All three compounds reduced phosphorylation of FAK, ERK1/2, and Akt. In addition, MMP-9 expression was downregulated by inhibiting FAK, ERK1/2, and Akt. Isothiocyanates-mediated inhibition of FAK phosphorylation suppressed phosphorylation of ERK1/2 and Akt in U2OS and A549 cells, along with the translocation of p65 and c-Fos, suggesting that isothiocyanates inhibit MMP-9 expression and cell invasion by blocking phosphorylation of FAK. Furthermore, isothiocyanates, abolished MMP-9 expression and tumor metastasis in vivo with the following efficacy: PEITC>BITC>SFN. Thus, isothiocyanates act as anti-metastatic compounds that suppress MMP-9 activity/expression by inhibiting NF-κB and AP-1 via suppression of the FAK/ERK and FAK/Akt signaling pathways.
Keywords: FAK; MMP-9; cancer invasion; isothiocyanates; metastasis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have declared that there are no conflicts of interest.
Figures


References
-
- Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS. 2011;278:16–27. - PubMed
-
- Chen YJ, Wei YY, Chen HT, Fong YC, Hsu CJ, Tsai CH, Hsu HC, Liu SH, Tang CH. Osteopontin increases migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cel Physiol. 2009;221:98–108. - PubMed
-
- Jablonska-Trypuc A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31:177–183. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

