Suppression of miR-708 inhibits the Wnt/β-catenin signaling pathway by activating DKK3 in adult B-all
- PMID: 28969056
- PMCID: PMC5609988
- DOI: 10.18632/oncotarget.19342
Suppression of miR-708 inhibits the Wnt/β-catenin signaling pathway by activating DKK3 in adult B-all
Abstract
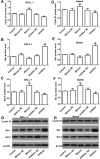
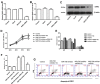
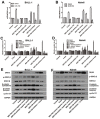
Inactivation of Dickkopf-3 (DKK3) is closely associated with a poor prognosis in various solid tumor and hematologic malignancies. Promoter hypermethylation is one potential cause of DKK3 inactivation. However, whether other mechanisms lead to DKK3 inactivation and the subsequent effects of these inactivations on cell proliferation and the Wnt signaling pathway in adult B acute lymphoblastic leukemia (B-ALL) remain unclear. In the present study, we found that low DKK3 expression levels were associated with high miR-708 expression and promoter hypermethylation in adult B-ALL. miR-708 was confirmed to directly decrease DKK3 expression in Nalm-6 and BALL-1 cells. Additionally, a miR-708 inhibitor decreased cell proliferation mainly through apoptosis and cell cycle arrest at the G1 phase, and these effects were eliminated by DKK3 siRNA treatment. Moreover, the demethylating agent 5-aza-2'-deoxycytidine (5-aza) decreased the methylation state of the DKK3 promoter based on methylation-specific PCR (MSP) and bisulfite genomic sequencing PCR (BSP), although this demethylation effect was not enhanced by the miR-708 inhibitor. The miR-708 inhibitor or 5-aza significantly increased DKK3 expression and decreased p-GSK3β, cyclin D1 and nuclear and cytoplasmic β-catenin protein expression, indicating that the Wnt/β-catenin signaling pathway was inhibited. These effects became more pronounced when the miR-708 inhibitor and 5-aza were used simultaneously. These findings provide greater insights into the mechanisms that increase DKK3 expression and suggest that a miR-708 inhibitor and 5-aza might be useful as targeted therapies for adult B-ALL.
Keywords: 5-aza-2’-deoxycytidine; DKK3; Wnt/β-catenin; adult B-acute lymphoblastic leukemia; miR-708.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no competing financial interests.
Figures




Similar articles
-
MYCN is a novel oncogenic target in adult B-ALL that activates the Wnt/β-catenin pathway by suppressing DKK3.J Cell Mol Med. 2018 Jul;22(7):3627-3637. doi: 10.1111/jcmm.13644. Epub 2018 Apr 19. J Cell Mol Med. 2018. PMID: 29673070 Free PMC article.
-
miR-214 ameliorates acute kidney injury via targeting DKK3 and activating of Wnt/β-catenin signaling pathway.Biol Res. 2018 Sep 4;51(1):31. doi: 10.1186/s40659-018-0179-2. Biol Res. 2018. PMID: 30180910 Free PMC article.
-
Wnt signalling in human breast cancer: expression of the putative Wnt inhibitor Dickkopf-3 (DKK3) is frequently suppressed by promoter hypermethylation in mammary tumours.Breast Cancer Res. 2008;10(5):R82. doi: 10.1186/bcr2151. Epub 2008 Sep 30. Breast Cancer Res. 2008. PMID: 18826564 Free PMC article.
-
WIF1 and DKK3 in prostate cancer: from molecular pathways to therapeutic targets: a narrative review.Transl Androl Urol. 2024 Nov 30;13(11):2601-2616. doi: 10.21037/tau-24-304. Epub 2024 Nov 28. Transl Androl Urol. 2024. PMID: 39698576 Free PMC article. Review.
-
Wogonin induced G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in human colorectal cancer carcinoma cells.Toxicology. 2013 Oct 4;312:36-47. doi: 10.1016/j.tox.2013.07.013. Epub 2013 Jul 30. Toxicology. 2013. PMID: 23907061 Review.
Cited by
-
LINC00514 drives osteosarcoma progression through sponging microRNA-708 and consequently increases URGCP expression.Aging (Albany NY). 2020 Apr 23;12(8):6793-6807. doi: 10.18632/aging.103043. Epub 2020 Apr 23. Aging (Albany NY). 2020. Retraction in: Aging (Albany NY). 2024 Aug 15;16(15):11771-11772. doi: 10.18632/aging.206066. PMID: 32325430 Free PMC article. Retracted.
-
Tumor-Suppressive MicroRNA-708 Targets Notch1 to Suppress Cell Proliferation and Invasion in Gastric Cancer.Oncol Res. 2018 Oct 17;26(9):1317-1326. doi: 10.3727/096504018X15179680859017. Epub 2018 Feb 14. Oncol Res. 2018. PMID: 29444743 Free PMC article.
-
Two Worlds Colliding: The Interplay Between Natural Compounds and Non-Coding Transcripts in Cancer Therapy.Front Pharmacol. 2021 Jul 6;12:652074. doi: 10.3389/fphar.2021.652074. eCollection 2021. Front Pharmacol. 2021. PMID: 34295245 Free PMC article. Review.
-
"Losing the Brakes"-Suppressed Inhibitors Triggering Uncontrolled Wnt/ß-Catenin Signaling May Provide a Potential Therapeutic Target in Elderly Acute Myeloid Leukemia.Curr Issues Mol Biol. 2023 Jan 9;45(1):604-613. doi: 10.3390/cimb45010040. Curr Issues Mol Biol. 2023. PMID: 36661526 Free PMC article.
-
MYCN is a novel oncogenic target in adult B-ALL that activates the Wnt/β-catenin pathway by suppressing DKK3.J Cell Mol Med. 2018 Jul;22(7):3627-3637. doi: 10.1111/jcmm.13644. Epub 2018 Apr 19. J Cell Mol Med. 2018. PMID: 29673070 Free PMC article.
References
-
- Kantarjian H, Thomas D, O'Brien S, Cortes J, Giles F, Jeha S, Bueso-Ramos CE, Pierce S, Shan J, Koller C, Beran M, Keating M, Freireich EJ. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. - PubMed
-
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

