Chronic non-freezing cold injury results in neuropathic pain due to a sensory neuropathy
- PMID: 28969380
- PMCID: PMC5841153
- DOI: 10.1093/brain/awx215
Chronic non-freezing cold injury results in neuropathic pain due to a sensory neuropathy
Abstract
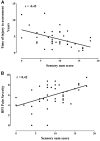
Non-freezing cold injury develops after sustained exposure to cold temperatures, resulting in tissue cooling but not freezing. This can result in persistent sensory disturbance of the hands and feet including numbness, paraesthesia and chronic pain. Both vascular and neurological aetiologies of this pain have been suggested but remain unproven. We prospectively approached patients referred for clinical assessment of chronic pain following non-freezing cold injury between 12 February 2014 and 30 November 2016. Of 47 patients approached, 42 consented to undergo detailed neurological evaluations including: questionnaires to detail pain location and characteristics, structured neurological examination, quantitative sensory testing, nerve conduction studies and skin biopsy for intraepidermal nerve fibre assessment. Of the 42 study participants, all had experienced non-freezing cold injury while serving in the UK armed services and the majority were of African descent (76.2%) and male (95.2%). Many participants reported multiple exposures to cold. The median time between initial injury and referral was 3.72 years. Pain was principally localized to the hands and the feet, neuropathic in nature and in all study participants associated with cold hypersensitivity. Clinical examination and quantitative sensory testing were consistent with a sensory neuropathy. In all cases, large fibre nerve conduction studies were normal. The intraepidermal nerve fibre density was markedly reduced with 90.5% of participants having a count at or below the 0.05 centile of published normative controls. Using the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain grading for neuropathic pain, 100% had probable and 95.2% definite neuropathic pain. Chronic non-freezing cold injury is a disabling neuropathic pain disorder due to a sensory neuropathy. Why some individuals develop an acute painful sensory neuropathy on sustained cold exposure is not yet known, but individuals of African descent appear vulnerable. Screening tools, such as the DN4 questionnaire, and treatment algorithms for neuropathic pain should now be used in the management of these patients.
Keywords: nerve conduction studies; neuropathic pain; peripheral nerve injury; sensory neuropathy; small fibre neuropathy.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Comment in
-
Non-freezing cold injury: a multi-faceted syndrome.Brain. 2018 Feb 1;141(2):e9. doi: 10.1093/brain/awx321. Brain. 2018. PMID: 29315359 No abstract available.
-
Reply: Non-freezing cold injury: a multi-faceted syndrome.Brain. 2018 Feb 1;141(2):e10. doi: 10.1093/brain/awx325. Brain. 2018. PMID: 29315377 No abstract available.
References
-
- Binder A, Stengel M, Maag R, Wasner G, Schoch R, Moosig F, et al.Pain in oxaliplatin-induced neuropathy—sensitisation in the peripheral and central nociceptive system. Eur J Cancer 2007; 43: 2658–63. - PubMed
-
- Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al.Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005; 114: 29–36. - PubMed
-
- Burgess JE, Macfarlane F. Retrospective analysis of the ethnic origins of male British army soldiers with peripheral cold weather injury. J R Army Med Corps 2009; 155: 11–15. - PubMed
-
- Buschbacher R, Orahlow N. Manual of nerve conduction studies. 2nd edn. New York, NY: Demos Medical Publishing; 2006.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

