IGF-1 as a Drug for Preterm Infants: A Step-Wise Clinical Development
- PMID: 28969546
- PMCID: PMC5824464
- DOI: 10.2174/1381612823666171002114545
IGF-1 as a Drug for Preterm Infants: A Step-Wise Clinical Development
Abstract
Background: Insulin-like growth factor 1 (IGF-1) is a mitogenic hormone involved in many processes such as growth, metabolism, angiogenesis and differentiation. After very preterm birth, energy demands increase while maternal supplies of nutrients and other factors are lost and the infant may become dependent on parenteral nutrition for weeks. Low postnatal IGF-1 concentrations in preterm infants are associated with poor weight gain, retinopathy of prematurity (ROP) and other morbidities. We will describe the process by which we aim to develop supplementation with recombinant human (rh) IGF-1 and its binding protein rhIGFBP-3 as a possible therapy to promote growth and maturation and reduce morbidities in extremely preterm infants.
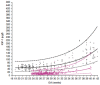
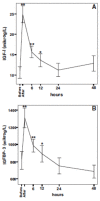
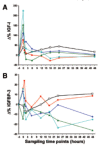
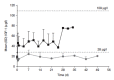
Methods: In order to calculate a dose of IGF-1 tolerated by neonates, a pharmacokinetic study of transfusion with fresh frozen plasma was performed, which provided a relatively low dose of IGF-1, (on average 1.4 µg/kg), that increased serum IGF-1 to levels close to those observed in fetuses and preterm infants of similar GAs. Thereafter, a Phase I 3 hours IV infusion of rhIGF-1/rhIGFBP-3 was conducted in 5 infants, followed by a Phase II study with four sections (A-D). In the Phase II, sections A-D studies, time on infusion increased and younger gestational ages were included.
Results: IV infusion increased IGF-1 but with short half-life (0.5h) implying a need for continuous infusion. In order to obtain in utero levels of IGF-I, the dose was increased from 100 to 250 µg/kg/24 h and the infusion was prolonged from 3 weeks postnatal age until a postmenstrual age of 29 weeks and 6 days.
Conclusion: The purpose has been to ensure high-quality research into the development of a new drug for preterm infants. We hope that our work will help to establish a new standard for the testing of medications for preterm infants.
Keywords: IGF-1; Preterm infant; drug development; pharmacokinetic; preterm morbidity..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures



References
-
- Hwa V.F.P., Derr M.A., Fiegerlova E., Rosenfeld R.G. 2013. - PubMed
-
- LeRoith D., Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007;3(3):302–310. - PubMed
-
- Hellstrom A., Engstrom E., Hard A.L., et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112(5):1016–1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
