TrueNTH sexual recovery study protocol: a multi-institutional collaborative approach to developing and testing a web-based intervention for couples coping with the side-effects of prostate cancer treatment in a randomized controlled trial
- PMID: 28969611
- PMCID: PMC5625773
- DOI: 10.1186/s12885-017-3652-3
TrueNTH sexual recovery study protocol: a multi-institutional collaborative approach to developing and testing a web-based intervention for couples coping with the side-effects of prostate cancer treatment in a randomized controlled trial
Abstract
Background: Over half of men who receive treatment for prostate suffer from a range of sexual problems that affect negatively their sexual health, sexual intimacy with their partners and their quality of life. In clinical practice, however, care for the sexual side effects of treatment is often suboptimal or unavailable. The goal of the current study is to test a web-based intervention to support the recovery of sexual intimacy of prostate cancer survivors and their partners after treatment.
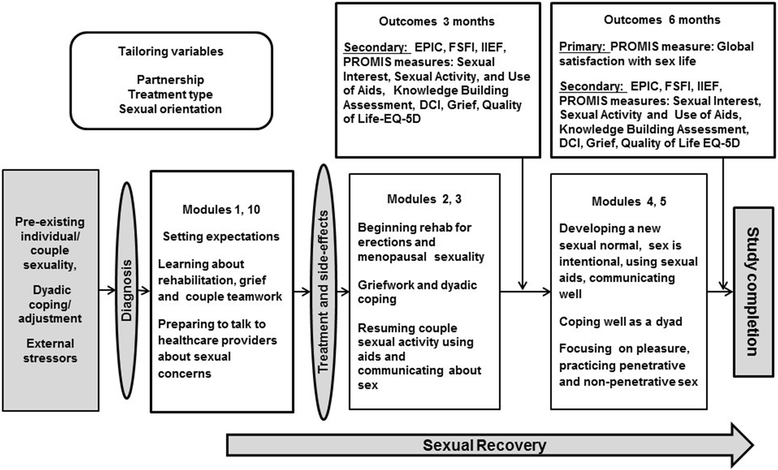
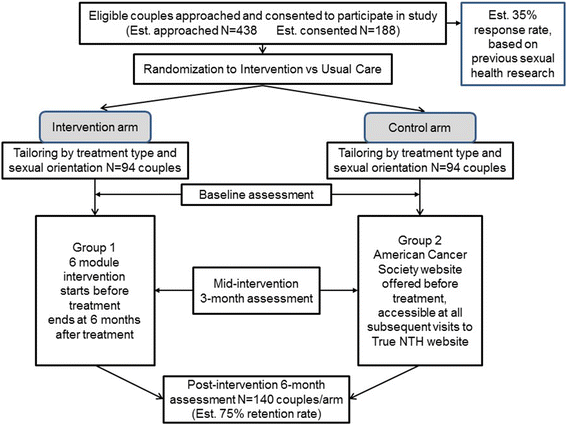
Methods: The study team developed an interactive, web-based intervention, tailored to type of treatment received, relationship status (partnered/non-partnered) and sexual orientation. It consists of 10 modules, six follow the trajectory of the illness and four are theme based. They address sexual side effects, rehabilitation, psychological impacts and coaching for self-efficacy. Each includes a video to engage participants, psychoeducation and activities completed by participants on the web. Tailored strategies for identified concerns are sent by email after each module. Six of these modules will be tested in a randomized controlled trial and compared to usual care. Men with localized prostate cancer with partners will be recruited from five academic medical centers. These couples (N = 140) will be assessed prior to treatment, then 3 months and 6 months after treatment. The primary outcome will be the survivors' and partners' Global Satisfaction with Sex Life, assessed by a Patient Reported Outcome Measure Information Systems (PROMIS) measure. Secondary outcomes will include interest in sex, sexual activity, use of sexual aids, dyadic coping, knowledge about sexual recovery, grief about the loss of sexual function, and quality of life. The impact of the intervention on the couple will be assessed using the Actor-Partner Interaction Model, a mixed-effects linear regression model able to estimate both the association of partner characteristics with partner and patient outcomes and the association of patient characteristics with both outcomes.
Discussion: The web-based tool represents a novel approach to addressing the sexual health needs of prostate cancer survivors and their partners that-if found efficacious-will improve access to much needed specialty care in prostate cancer survivorship.
Trial registration: Clinicaltrials.gov registration # NCT02702453 , registered on March 3, 2016.
Keywords: Prostate cancer sexual recovery cancer survivorship intervention.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the following institutional review boards:
University of Michigan: Medical School Institutional Review Board (IRBMED).
Emory University: Institutional Review Board (IRB).
Memorial Sloan Kettering Cancer Center: Institutional Review Board/Privacy Board-B.
University of California Los Angeles: Office of Human Research Protection Program (OHRPP).
Johns Hopkins University: Office of Human Subjects Research Institutional Review Boards.
All participants must sign informed consent before enrolling in the study.
Consent for publication
Consent for publication is not needed as no individual patient data or images are involved in this research.
Competing interests
◦ Dr. Wittmann has Movember Foundation research funding.
◦ Dr. Coward is a Consultant to Coloplast Corporation
◦ Dr. Koontz receives research funding from Janssen Pharmaceuticals and royalties from UpToDate®. She acts on an Advisory Board for Blue Earth Pharmaceuticals
◦ Dr. Lowe is the Chief Executive Officer of Prostate Cancer Foundation of Australia and consults to the Menzies Health Institute Queensland, Griffith University and ANZUP Cancer Trails Group Limited
◦ Dr. Mulhall has research funding from Pfizer, Inc. and is a Consultant to the following entitites: Eli Lilly and Co., Alliance for Fertility Preservation, Nexmed, Absorption Pharmaceuticals, AMS, Meda, Vivus, and has a leadership role with the Association of Peyronie’s Disease Advocates
◦ Ms. Paich is a US Project Manager for TrueNTH Movember Foundation
◦ Dr. Skolarus is supported by a VA HSR&D Career Development Award - 2 (CDA 12–171) and receives royalties from UpToDate®.
◦ Dr. Saigal is WiserCare Cofounder and Board Member
◦ Dr. Walsh is a Consultant to Coloplast Corporation and Boston Scientific
◦ All other authors have no relevant disclosures
◦ The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Evaluating a couple-based intervention addressing sexual concerns for breast cancer survivors: study protocol for a randomized controlled trial.Trials. 2020 Feb 12;21(1):173. doi: 10.1186/s13063-019-3975-2. Trials. 2020. PMID: 32051002 Free PMC article.
-
Exploring the feasibility and acceptability of couple-based psychosexual support following prostate cancer surgery: study protocol for a pilot randomised controlled trial.Trials. 2014 May 24;15:183. doi: 10.1186/1745-6215-15-183. Trials. 2014. PMID: 24886676 Free PMC article. Clinical Trial.
-
TrueNTH Sexual Recovery Intervention for couples coping with prostate cancer: Randomized controlled trial results.Cancer. 2022 Apr 1;128(7):1513-1522. doi: 10.1002/cncr.34076. Epub 2022 Jan 5. Cancer. 2022. PMID: 34985771 Clinical Trial.
-
Communication and intimacy-enhancing interventions for men diagnosed with prostate cancer and their partners.J Sex Med. 2013 Feb;10 Suppl 1(0 1):127-32. doi: 10.1111/jsm.12049. J Sex Med. 2013. PMID: 23387918 Free PMC article. Review.
-
Sexual Health Recovery For Prostate Cancer Survivors: The Proposed Role Of Acceptance And Mindfulness-Based Interventions.Sex Med Rev. 2019 Oct;7(4):627-635. doi: 10.1016/j.sxmr.2019.03.001. Epub 2019 Apr 24. Sex Med Rev. 2019. PMID: 31029619 Review.
Cited by
-
Sexual Health Outcomes of Adolescent and Young Adult Colorectal Cancer Survivors and Their Partners: Protocol of a Dyadic Mixed Methods Study.JMIR Res Protoc. 2023 Mar 23;12:e41831. doi: 10.2196/41831. JMIR Res Protoc. 2023. PMID: 36951909 Free PMC article.
-
Post-Treatment Adverse Health Correlates among Prostate Cancer Survivors in a Sample of Men Residing in Atlantic Canada.Curr Oncol. 2021 Jul 25;28(4):2812-2822. doi: 10.3390/curroncol28040246. Curr Oncol. 2021. PMID: 34436012 Free PMC article.
-
Dyadic Coping in Couples Facing Chronic Physical Illness: A Systematic Review.Front Psychol. 2021 Oct 25;12:722740. doi: 10.3389/fpsyg.2021.722740. eCollection 2021. Front Psychol. 2021. PMID: 34759866 Free PMC article.
-
An online Sexual Health and Rehabilitation eClinic (TrueNTH SHAReClinic) for prostate cancer patients: a feasibility study.Support Care Cancer. 2022 Feb;30(2):1253-1260. doi: 10.1007/s00520-021-06510-4. Epub 2021 Aug 31. Support Care Cancer. 2022. PMID: 34463836 Free PMC article.
-
A qualitative analysis of sexual transformation in Japanese women after ovarian cancer treatment.Asia Pac J Oncol Nurs. 2024 Jan 19;11(4):100381. doi: 10.1016/j.apjon.2024.100381. eCollection 2024 Apr. Asia Pac J Oncol Nurs. 2024. PMID: 38495644 Free PMC article.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical