Effects of dietary supplementation with a standardized aqueous extract of Terminalia chebula fruit (AyuFlex®) on joint mobility, comfort, and functional capacity in healthy overweight subjects: a randomized placebo-controlled clinical trial
- PMID: 28969626
- PMCID: PMC5625793
- DOI: 10.1186/s12906-017-1977-8
Effects of dietary supplementation with a standardized aqueous extract of Terminalia chebula fruit (AyuFlex®) on joint mobility, comfort, and functional capacity in healthy overweight subjects: a randomized placebo-controlled clinical trial
Abstract
Background: Joint and connective tissue integrity, comfort and function are paramount to optimal performance in exercise, recreational and occupational activities. The fruit of Terminalia chebula has been used extensively in various traditional health systems for different ailments, with additional preclinical and clinical data demonstrating antioxidant and anti-inflammatory potential. The aim of this study was to evaluate the effects of a standardized aqueous extract of Terminalia chebula fruit (AyuFlex®) dietary supplementation on joint mobility, comfort, and functional capacity in healthy overweight subjects.
Methods: One-hundred and five (105) overweight, apparently healthy male and female subjects (35-70 years of age) were pre-screened and randomized to one of three groups for 84 days: placebo, AyuFlex1 (250 mg twice daily) or AyuFlex2 (500 mg twice daily) in a randomized, double-blind, placebo-controlled design. A two-week placebo lead-in period was used to improve data quality/validity. All subjects had no knee joint discomfort at rest, but experienced knee joint discomfort only with activity/exercise of at least 30 on 100 mm Visual Analog Scale (VAS). Primary outcome measures included symptoms of joint health and function as measured by modified-Knee Injury & Osteoarthritis Outcomes Score (mKOOS) global & modified-Western Ontario and McMaster Universities Arthritis Index (mWOMAC) subscales (discomfort, stiffness and function). Secondary outcomes included VAS questionnaires on overall/whole-body joint health, low back health, knee mobility, willingness and ability to exercise, 6-min walk test for distance and range of motion (ROM) of pain-free knee flexion/extension. Tertiary outcome measures included inflammatory (high sensitivity C-reactive protein (hsCRP), tumor necrosis factor (TNF)-α) and extracellular matrix (ECM)/Connective Tissue (COMP) biomarkers, and safety (vital signs and blood markers) & tolerability (Adverse Event (AE)/ side effect profiles).
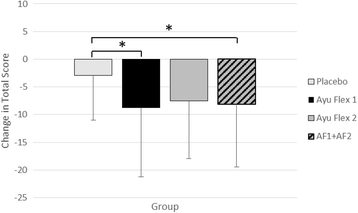
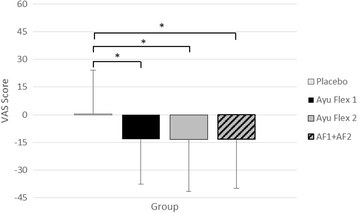
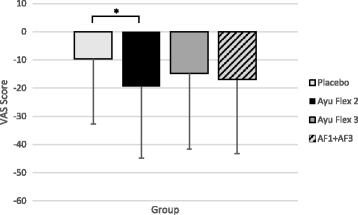
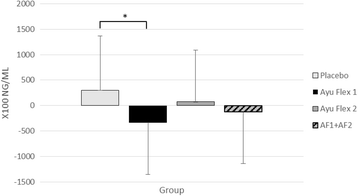
Results: Compared to placebo, at day 84 AyuFlex® treatment significantly: 1) improved mKOOS global scores in AyuFlex1 + AyuFlex2 (P = 0.023), and improved total and physical function subscale of mWOMAC relative to baseline, 2) improved VAS scores for Knee Discomfort with activity/exercise in AyuFlex1 + AyuFlex2 (P = 0.001) relative to baseline, 3) improved VAS scores for whole-body joint function in AyuFlex1 + AyuFlex2 (P < 0.029) relative to baseline, 4) improved VAS score for decreased knee joint soreness following leg extension challenge for AyuFlex1 (P = 0.022) and AyuFlex2 (P = 0.043) relative to baseline, 5) improved 6-min walk performance distance covered (P = 0.047) and VAS discomfort (P = 0.026) post-6 min walk in AyuFlex1 + AyuFlex2 relative to baseline, 6) and tended to decrease COMP levels in AyuFlex1 + AyuFLex2 (P = 0.104) relative to baseline. All biomarkers of safety remained within normative limits during the study. Low back health tended to improve in the AyuFlex1 and AyuFlex2 group, but failed to reach significance relative to placebo group.
Conclusions: AyuFlex® improved mKOOS global scores, knee joint discomfort with activity/exercise, 6-min walk test distance covered and discomfort post-6 min walk test, overall whole-body joint function, knee soreness following leg extension resistance exercise in a healthy, overweight population, without AE. Differences between 250 mg/BID and 500 mg/BID were non-significant for most of the outcome measures, validating the efficacy of the lower dose.
Trial registration: ClinicalTrials.gov identifier NCT02589249 ; October 26, 2015.
Keywords: Anti-inflammatory; AyuFlex; Cartilage oligomeric protein; Chebulagic acids; Chebulinic acid; Connective tissue; Exercise capacity; Joint health; Joint mobility; Knee; Terminalia chebula; Western Ontario McMaster universities arthritis index.
Conflict of interest statement
Ethics approval and consent to participate
The protocol was reviewed, and all procedures approved by an independent, FDA-audited, Institutional Review Board (IntegReview, Austin, TX; October 29th, 2015) and the study was registered at
Competing interests
Dr. Lopez has no conflict in terms of financial or business interests related to this product. Dr. Lopez is an officer and member of The Center for Applied Health Sciences, a privately held contract research organization that has received external funding from companies that does business in the dietary supplement, natural products, medical foods and functional foods and beverages industry. He is co-founder and member of Supplement Safety Solutions, LLC., serving as an independent consultant for regulatory compliance, safety surveillance and Nutravigilance to companies in the dietary supplement and functional foods industry, but not Natreon, Inc., the sponsor of the current research. Dr. Lopez is also co-inventor on multiple patent applications within the field of dietary supplements, applied nutrition and bioactive compounds.
Dr. Ziegenfuss has no conflict in terms of financial or business interests related to this product. Dr. Ziegenfuss has received grants and contracts to conduct research on dietary supplements; has served as a paid consultant for industry; has received honoraria for speaking at conferences and writing lay articles about sports nutrition ingredients (but not the product investigated in this study); receives royalties from the sale of several sports nutrition products (but not from Natreon, Inc); and has served as an expert witness on behalf of the plaintiff and defense in cases involving dietary supplements. Dr. Ziegenfuss is also co-inventor on multiple patent applications within the field of dietary supplements, applied nutrition and bioactive compounds.
Dr. Kedia, Mr. Habowski, Ms. Sandrock, Ms. Raub and Mr. Bruno all report no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures







References
-
- American Academy of Orthopaedic Surgeons: Osteoarthritis. http://orthoinfo.aaos.org/topic.cfm?topic=a00227 (2007). Accessed 8 Nov 2016.
-
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F, National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

