Impact of mesenchymal stem cells' secretome on glioblastoma pathophysiology
- PMID: 28969635
- PMCID: PMC5625623
- DOI: 10.1186/s12967-017-1303-8
Impact of mesenchymal stem cells' secretome on glioblastoma pathophysiology
Abstract
Background: Glioblastoma (GBM) is a highly aggressive primary brain cancer, for which curative therapies are not available. An emerging therapeutic approach suggested to have potential to target malignant gliomas has been based on the use of multipotent mesenchymal stem cells (MSCs), either unmodified or engineered to deliver anticancer therapeutic agents, as these cells present an intrinsic capacity to migrate towards malignant tumors. Nevertheless, it is still controversial whether this innate tropism of MSCs towards the tumor area is associated with cancer promotion or suppression. Considering that one of the major mechanisms by which MSCs interact with and modulate tumor cells is via secreted factors, we studied how the secretome of MSCs modulates critical hallmark features of GBM cells.
Methods: The effect of conditioned media (CM) from human umbilical cord perivascular cells (HUCPVCs, a MSC population present in the Wharton's jelly of the umbilical cord) on GBM cell viability, migration, proliferation and sensitivity to temozolomide treatment of U251 and SNB-19 GBM cells was evaluated. The in vivo chicken chorioallantoic membrane (CAM) assay was used to evaluate the effect of HUCPVCs CM on tumor growth and angiogenesis. The secretome of HUCPVCs was characterized by proteomic analyses.
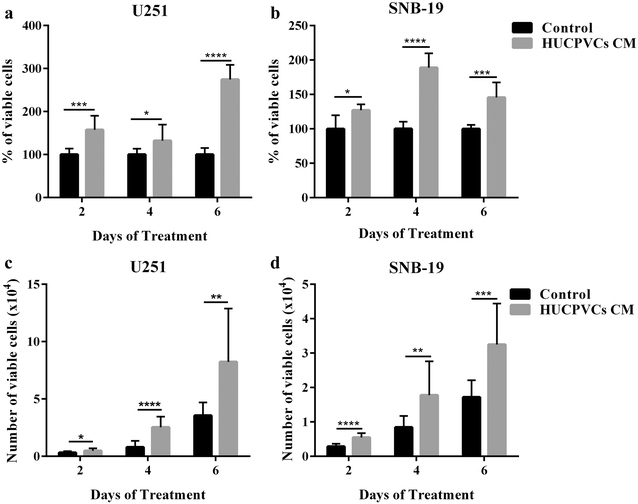
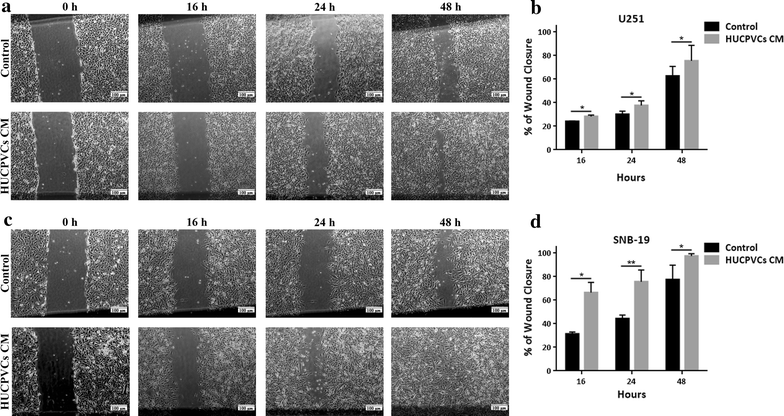
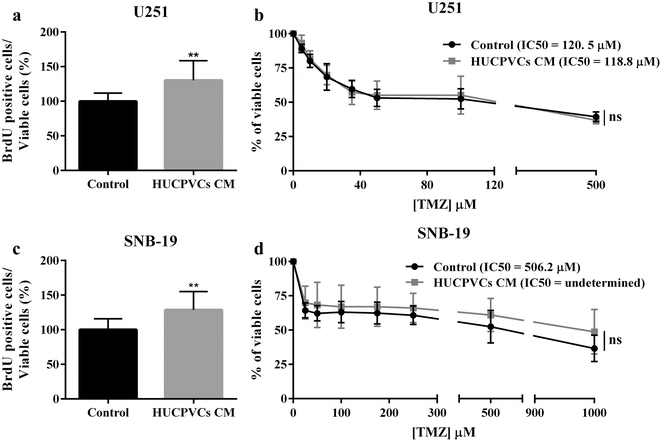
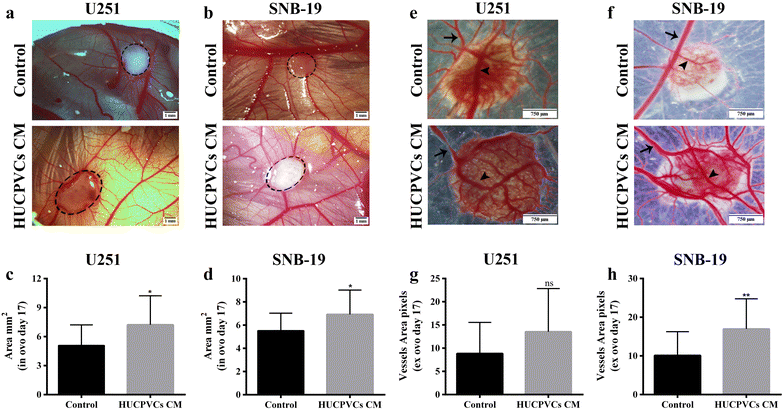
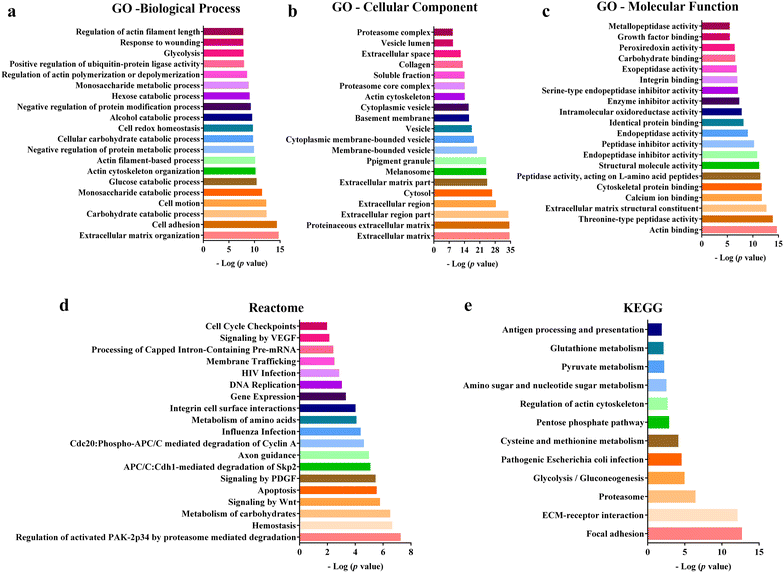
Results: We found that both tested GBM cell lines exposed to HUCPVCs CM presented significantly higher cellular viability, proliferation and migration. In contrast, resistance of GBM cells to temozolomide chemotherapy was not significantly affected by HUCPVCs CM. In the in vivo CAM assay, CM from HUCPVCs promoted U251 and SNB-19 tumor cells growth. Proteomic analysis to characterize the secretome of HUCPVCs identified several proteins involved in promotion of cell survival, proliferation and migration, revealing novel putative molecular mediators for the effects observed in GBM cells exposed to HUCPVCs CM.
Conclusions: These findings provide novel insights to better understand the interplay between GBM cells and MSCs, raising awareness to potential safety issues regarding the use of MSCs as stem-cell based therapies for GBM.
Keywords: Conditioned media; Glioblastoma; Human umbilical cord perivascular cells; Mesenchymal stem cells; Migration; Proliferation; Proteomics; Secretome; Viability.
Figures
Similar articles
-
Analysis of the safety of mesenchymal stromal cells secretome for glioblastoma treatment.Cytotherapy. 2016 Jul;18(7):828-37. doi: 10.1016/j.jcyt.2016.03.299. Epub 2016 May 17. Cytotherapy. 2016. PMID: 27210718
-
Unveiling the effects of the secretome of mesenchymal progenitors from the umbilical cord in different neuronal cell populations.Biochimie. 2013 Dec;95(12):2297-303. doi: 10.1016/j.biochi.2013.06.028. Epub 2013 Jul 10. Biochimie. 2013. PMID: 23851197
-
Tropism of mesenchymal stem cell toward CD133+ stem cell of glioblastoma in vitro and promote tumor proliferation in vivo.Stem Cell Res Ther. 2018 Nov 9;9(1):310. doi: 10.1186/s13287-018-1049-0. Stem Cell Res Ther. 2018. PMID: 30413179 Free PMC article.
-
Cross talk between mesenchymal and glioblastoma stem cells: Communication beyond controversies.Stem Cells Transl Med. 2020 Nov;9(11):1310-1330. doi: 10.1002/sctm.20-0161. Epub 2020 Jun 15. Stem Cells Transl Med. 2020. PMID: 32543030 Free PMC article. Review.
-
Proteomic techniques for characterisation of mesenchymal stem cell secretome.Biochimie. 2013 Dec;95(12):2196-211. doi: 10.1016/j.biochi.2013.07.015. Epub 2013 Jul 20. Biochimie. 2013. PMID: 23880644 Review.
Cited by
-
Rationale for the Use of Radiation-Activated Mesenchymal Stromal/Stem Cells in Acute Respiratory Distress Syndrome.Cells. 2020 Sep 2;9(9):2015. doi: 10.3390/cells9092015. Cells. 2020. PMID: 32887260 Free PMC article. Review.
-
Benign non-immune cells in tumor microenvironment.Front Immunol. 2025 Apr 3;16:1561577. doi: 10.3389/fimmu.2025.1561577. eCollection 2025. Front Immunol. 2025. PMID: 40248695 Free PMC article. Review.
-
The impact of stem cells in neuro-oncology: applications, evidence, limitations and challenges.Acta Biomed. 2020 Jun 30;91(7-S):51-60. doi: 10.23750/abm.v91i7-S.9955. Acta Biomed. 2020. PMID: 32608375 Free PMC article. Review.
-
Enhancing the Bystander and Abscopal Effects to Improve Radiotherapy Outcomes.Front Oncol. 2020 Jan 8;9:1381. doi: 10.3389/fonc.2019.01381. eCollection 2019. Front Oncol. 2020. PMID: 31970082 Free PMC article. Review.
-
Secretome of Dental Pulp-Derived Stem Cells Reduces Inflammation and Proliferation of Glioblastoma Cells by Deactivating Mapk-Akt Pathway.Dis Res. 2023 Dec;3(2):74-86. doi: 10.54457/DR.202302006. Epub 2023 Jul 19. Dis Res. 2023. PMID: 38213319 Free PMC article.
References
-
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

