A randomized, controlled, double-blind, crossover trial of triheptanoin in alternating hemiplegia of childhood
- PMID: 28969699
- PMCID: PMC5625655
- DOI: 10.1186/s13023-017-0713-2
A randomized, controlled, double-blind, crossover trial of triheptanoin in alternating hemiplegia of childhood
Abstract
Background: Based on the hypothesis of a brain energy deficit, we investigated the safety and efficacy of triheptanoin on paroxysmal episodes in patients with alternating hemiplegia of childhood due to ATP1A3 mutations.
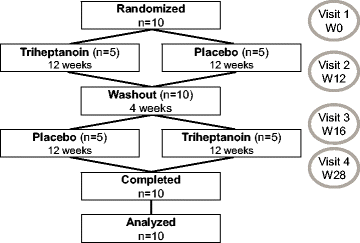
Methods: We conducted a randomized, double-blind, placebo-controlled crossover study of triheptanoin, at a target dose corresponding to 30% of daily calorie intake, in ten patients with alternating hemiplegia of childhood due to ATP1A3 mutations. Each treatment period consisted of a 12-week fixed-dose phase, separated by a 4-week washout period. The primary outcome was the total number of paroxysmal events. Secondary outcomes included the number of paroxysmal motor-epileptic events; a composite score taking into account the number, severity and duration of paroxysmal events; interictal neurological manifestations; the clinical global impression-improvement scale (CGI-I); and safety parameters. The paired non-parametric Wilcoxon test was used to analyze treatment effects.
Results: In an intention-to-treat analysis, triheptanoin failed to reduce the total number of paroxysmal events (p = 0.646), including motor-epileptic events (p = 0.585), or the composite score (p = 0.059). CGI-I score did not differ between triheptanoin and placebo periods. Triheptanoin was well tolerated.
Conclusions: Triheptanoin does not prevent paroxysmal events in Alternating hemiplegia of childhood. We show the feasibility of a randomized placebo-controlled trial in this setting.
Trial registration: The study has been registered with clinicaltrials.gov ( NCT002408354 ) the 03/24/2015.
Trial registration: ClinicalTrials.gov NCT02408354.
Keywords: Alternating hemiplegia of childhood; Crossover trial; Triheptanoin.
Conflict of interest statement
Authors’ information
Sandrine Leroy carried out the biostatistical analysis.
Ethics approval and consent to participate
The study protocol was approved by a local ethics committee (CPP Paris VI), and written informed consent was obtained from all the participants or their legal guardians. The study has been registered with clinicaltrials.gov (NCT002408354) the 03/24/2015.
Consent for publication
Not applicable (manuscript contains no individual person’s data).
Competing interests
Elodie Hainque, Samantha Caillet, Sandrine Leroy, Constance Flamand-Roze, Isaac Adanyeguh, Fanny Charbonnier-Beaupel, Maryvonne Retail, Benjamin Le Toullec, Anne Roubergue, Mariana Atencio, Sophie Rivaud-Péchoux, Vanessa Brochard, Florence Habarou, Chris Ottolenghi, Florence Cormier, Mohamed Doulazmi and Marta Ruiz declare that they have no competing interests.
Aurélie Méneret has received travel funding from Zambon.
Jean-Christophe Corvol has stock options at B&A Therapeutics; has received research grants from the French Ministry of Health, ANR, Michael J Fox Foundation, Actelion and Ipsen; participated in scientific advisory boards for BMS, Zambon, Pfizer, Amarantus, Abbvie and Clevexel; and received travel grants from Abbvie and Teva.
Fanny Mochel holds a patent on the use of triheptanoin in Huntington disease and GLUT1 deficiency syndrome. She has received research support from Ultragenyx and honorarium on advisory boards from Ultragenyx and AlfaSigma.
Marie Vidailhet has received grant from Merz and travel grants from MDS and EAN.
Emmanuel Roze has received research support from Merz-Pharma, Orkyn, Aguettant, IP santé, Ultragenix and UCB pharma; served on scientific advisory boards for Orkyn, Ultragenix, Retrophin and Merz-pharma; received speech honoraria from Orkyn, Aguettant, Merz-Pharma and Ultragenix; and received travel funding from the Dystonia Coalition, the Dystonia Medical Research Foundation, the Movement Disorders Society, and the European Academy of Neurology.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Similar articles
-
Triheptanoin Did Not Show Benefit versus Placebo for the Treatment of Paroxysmal Movement Disorders in Glut1 Deficiency Syndrome: Results of a Randomized Phase 3 Study.Mov Disord. 2024 Aug;39(8):1386-1396. doi: 10.1002/mds.29822. Epub 2024 May 9. Mov Disord. 2024. PMID: 38725190 Clinical Trial.
-
Management of Alternating Hemiplegia of Childhood: A Review.Pediatr Neurol. 2020 Feb;103:12-20. doi: 10.1016/j.pediatrneurol.2019.10.003. Epub 2019 Nov 1. Pediatr Neurol. 2020. PMID: 31836335 Review.
-
A double-blind, placebo-controlled trial of triheptanoin in adult polyglucosan body disease and open-label, long-term outcome.J Inherit Metab Dis. 2018 Sep;41(5):877-883. doi: 10.1007/s10545-017-0103-x. Epub 2017 Nov 6. J Inherit Metab Dis. 2018. PMID: 29110179 Clinical Trial.
-
Alternating hemiplegia of childhood: evolution over time and mouse model corroboration.Brain Commun. 2021 Jun 4;3(3):fcab128. doi: 10.1093/braincomms/fcab128. eCollection 2021. Brain Commun. 2021. PMID: 34396101 Free PMC article.
-
Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment--A retrospective chart review.Mol Genet Metab. 2015 Sep-Oct;116(1-2):53-60. doi: 10.1016/j.ymgme.2015.06.006. Epub 2015 Jun 18. Mol Genet Metab. 2015. PMID: 26116311 Free PMC article. Review.
Cited by
-
Navigating the Complexity of Alternating Hemiplegia in Childhood: A Comprehensive Review.Rambam Maimonides Med J. 2024 Jul 30;15(3):e0015. doi: 10.5041/RMMJ.10529. Rambam Maimonides Med J. 2024. PMID: 39088707 Free PMC article. Review.
-
Triheptanoin: First Approval.Drugs. 2020 Oct;80(15):1595-1600. doi: 10.1007/s40265-020-01399-5. Drugs. 2020. PMID: 32897506 Free PMC article. Review.
-
No effect of triheptanoin on exercise performance in McArdle disease.Ann Clin Transl Neurol. 2019 Oct;6(10):1949-1960. doi: 10.1002/acn3.50863. Epub 2019 Sep 14. Ann Clin Transl Neurol. 2019. PMID: 31520525 Free PMC article. Clinical Trial.
-
Heptanoic and medium branched-chain fatty acids as anaplerotic treatment for medium chain acyl-CoA dehydrogenase deficiency.Mol Genet Metab. 2023 Nov;140(3):107689. doi: 10.1016/j.ymgme.2023.107689. Epub 2023 Aug 25. Mol Genet Metab. 2023. PMID: 37660571 Free PMC article.
-
Medium branched chain fatty acids improve the profile of tricarboxylic acid cycle intermediates in mitochondrial fatty acid β-oxidation deficient cells: A comparative study.J Inherit Metab Dis. 2022 May;45(3):541-556. doi: 10.1002/jimd.12480. Epub 2022 Feb 2. J Inherit Metab Dis. 2022. PMID: 35076099 Free PMC article.
References
-
- Rosewich H, Thiele H, Ohlenbusch A, Maschke U, Altmüller J, Frommolt P, et al. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol. 2012;11(9):764–773. doi: 10.1016/S1474-4422(12)70182-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical