Community-based trial of annual versus biannual single-dose ivermectin plus albendazole against Wuchereria bancrofti infection in human and mosquito populations: study protocol for a cluster randomised controlled trial
- PMID: 28969715
- PMCID: PMC5625710
- DOI: 10.1186/s13063-017-2196-9
Community-based trial of annual versus biannual single-dose ivermectin plus albendazole against Wuchereria bancrofti infection in human and mosquito populations: study protocol for a cluster randomised controlled trial
Abstract
Background: The Global Programme for the Elimination of Lymphatic Filariasis (GPELF) has been in operation since the year 2000, with the aim of eliminating the disease by the year 2020, following five to six rounds of effective annual mass drug administration (MDA). The treatment regimen is ivermectin (IVM) in combination with diethylcarbamazine (DEC) or albendazole (ALB). In Ghana, MDA has been undertaken since 2001. While the disease has been eliminated in many areas, transmission has persisted in some implementation units that had experienced 15 or more rounds of MDA. Thus, new intervention strategies could eliminate residual infection in areas of persistent transmission and speed up the lymphatic filariasis (LF)-elimination process. This study, therefore, seeks to test the hypothesis that biannual treatment of LF-endemic communities will accelerate the interruption of LF in areas of persistent transmission.
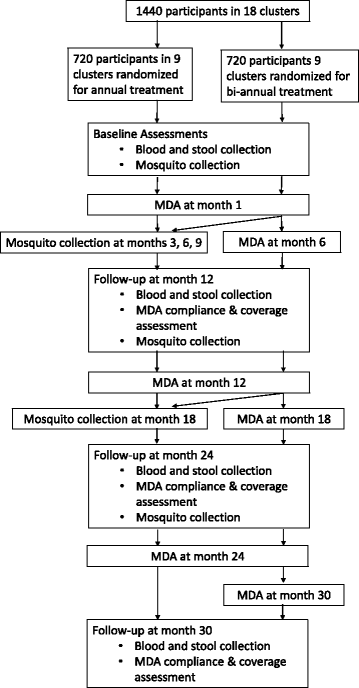
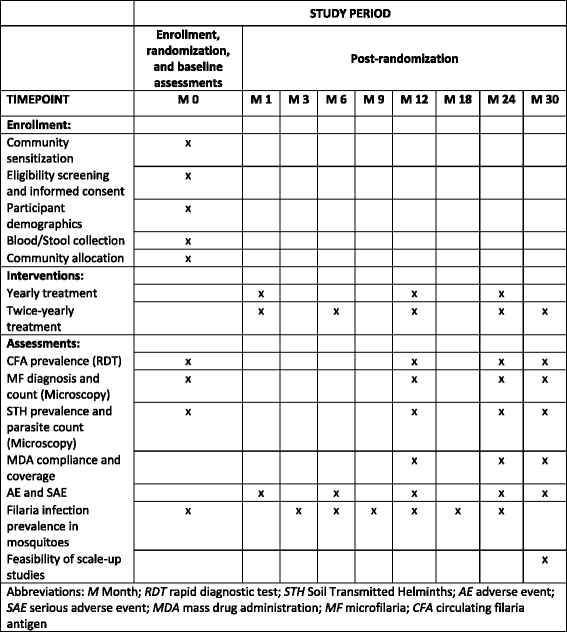
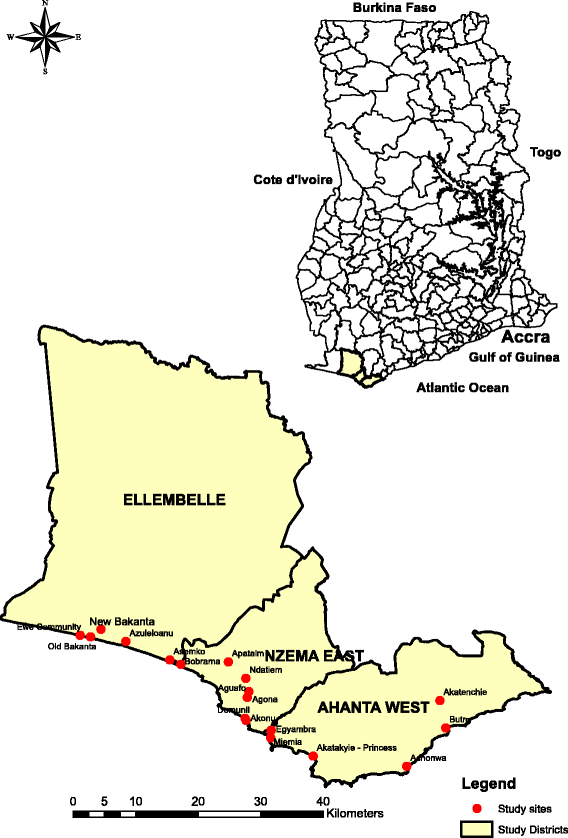
Methods: A cluster randomised trial will be implemented in LF-endemic communities in Ghana. The interventions will be yearly or twice-yearly MDA delivered to entire endemic communities. Allocation to study group will be by clusters identified using the prevalence of LF. Clusters will be randomised to one of two groups: receiving either (1) annual treatment with IVM + ALB or (2) annual MDA with IVM + ALB, followed by an additional MDA 6 months later. The primary outcome measure is the prevalence of LF infection, assessed by four cross-sectional surveys. Entomological assessments will also be undertaken to evaluate the transmission intensity of the disease in the study clusters. Costs and cost-effectiveness will be evaluated. Among a random subsample of participants, microfilaria prevalence will be assessed longitudinally. A nested process evaluation, using semi-structured interviews, focus group discussions and a stakeholder analysis, will investigate the community acceptability, feasibility and scale-up of each delivery system.
Discussion: It is expected that this study will add to the existing evidence on the need for alternative intervention strategies for the elimination of LF in Ghana and in other African countries that are facing similar challenges or are at the beginning of their LF-elimination programmes.
Trial registration: ClinicalTrials.gov, ID: NCT03036059 . Registered on 26 January 2017. Pan African Clinical Trials Registry, ID: PACTR201702002012425 . Registered on 23 February 2017.
Keywords: Biannual treatment; Increased treatment frequency; Lymphatic filariasis; Twice-yearly treatment.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval of the study has been obtained from the Ethics Committee of the Ghana Health Service (GHS-ERC: 04112/2016), and the IRB of the Noguchi Memorial Institute for Medical Research (CPN 062/16-17) with Federal Wide Assurance Registration (FWA 00001824). An Informed Consent Form has to be signed by all study participants who agree to participate in the current study.
Consent for publication
Written informed consent will be obtained from all study participants. Publications resulting from this study will not contain participant identifying information or images. Informed Consent Forms are held by the authors and available for review, upon request.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Simonsen PE, Magesa SM, Dunyo SK, Malecela-Lazaro MN, Michael E. The effect of single dose ivermectin alone or in combination with albendazole on Wuchereria bancrofti infection in primary school children in Tanzania. Trans R Soc Trop Med Hyg. 2004;98(8):462–72. doi: 10.1016/j.trstmh.2003.12.005. - DOI - PubMed
-
- Simonsen PE, Meyrowitsch DW, Mukoko DA, Pedersen EM, Malecela-Lazaro MN, Rwegoshora RT, et al. The effect of repeated half-yearly diethylcarbamazine mass treatment on Wuchereria bancrofti infection and transmission in two East African communities with different levels of endemicity. Am J Trop Med Hyg. 2004;70(1):63–71. - PubMed
-
- World Health Organisation . Annual Report of the Global Programme to Eliminate Lymphatic Filariasis. Geneva: WHO; 2002.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous