Bio-Heat Is a Key Environmental Driver Shaping the Microbial Community of Medium-Temperature Daqu
- PMID: 28970223
- PMCID: PMC5691423
- DOI: 10.1128/AEM.01550-17
Bio-Heat Is a Key Environmental Driver Shaping the Microbial Community of Medium-Temperature Daqu
Abstract
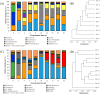
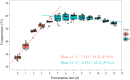
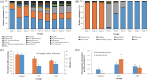
"Daqu" is a saccharifying and fermenting agent commonly used in the traditional solid-state fermentation industry (e.g., baijiu and vinegar). The patterns of microbial community succession and flavor formation are highly similar among batches, yet the mechanisms promoting temporal succession in the Daqu microbial ecology remain unclear. Here, we first correlated temporal profiles of microbial community succession with environmental variables (temperature, moisture, and titratable acidity) in medium temperature Daqu (MT-Daqu) throughout fermentation. Temperature dynamics significantly correlated (P < 0.05) with the quick succession of MT-Daqu microbiota in the first 12 d of fermentation, while the community structure was relatively stable after 12 d. Then, we explored the effect of temperature on the MT-Daqu community assembly. In the first 4 d of fermentation, the rapid propagation of most bacterial taxa and several fungal taxa, including Candida, Wickerhamomyces, and unclassified Dipodascaceae and Saccharomycetales species, significantly increased MT-Daqu temperature to 55°C. Subsequently, sustained bio-heat generated by microbial metabolism (53 to 56°C) within MT-Daqu inhibited the growth of most microbes from day 4 to day 12, while thermotolerant taxa, including Bacillus, unclassified Streptophyta, Weissella, Thermoactinomyces, Thermoascus, and Thermomyces survived or kept on growing. Furthermore, temperature as a major driving force on the shaping of MT-Daqu microbiota was validated. Lowering the fermentation temperature by placing the MT-Daqu in a 37°C incubator resulted in decreased relative abundances of thermotolerant taxa, including Bacillus, Thermoactinomyces, and Thermoascus, in the MT-Daqu microbiota. This study revealed that bio-heat functioned as a primary endogenous driver promoting the formation of functional MT-Daqu microbiota.IMPORTANCE Humans have mastered the Daqu preparation technique of cultivating functional microbiota on starchy grains over thousands of years, and it is well known that the metabolic activity of these microbes is key to the flavor production of Chinese baijiu. The pattern of microbial community succession and flavor formation remains highly similar between batches, yet mechanistic insight into these patterns and into microbial population fidelity to specific environmental conditions remains unclear. Our study revealed that bio-heat was generated within Daqu bricks in the first 4 d of fermentation, concomitant with rapid microbial propagation and metabolism. The sustained bio-heat may then function as a major endogenous driving force promoting the formation of the MT-Daqu microbiota from day 4 to day 12. The bio-heat-driven growth of thermotolerant microorganisms might contribute to the formation of flavor metabolites. This study provides useful information for the temperature-based modulation of microbiota function during the fermentation of Daqu.
Keywords: Daqu; bio-heat; metagenomics; microbial communities.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
Microbial community of Nongxiangxing daqu during storage: microbial succession, assembly mechanisms and metabolic functions.J Sci Food Agric. 2025 May;105(7):3665-3678. doi: 10.1002/jsfa.14118. Epub 2025 Jan 17. J Sci Food Agric. 2025. PMID: 39821324
-
Comparison of microbial community and metabolites in spontaneous fermentation of two types Daqu starter for traditional Chinese vinegar production.J Biosci Bioeng. 2019 Sep;128(3):307-315. doi: 10.1016/j.jbiosc.2019.03.011. Epub 2019 Apr 22. J Biosci Bioeng. 2019. PMID: 31023532
-
Bacteria and filamentous fungi running a relay race in Daqu fermentation enable macromolecular degradation and flavor substance formation.Int J Food Microbiol. 2023 Apr 2;390:110118. doi: 10.1016/j.ijfoodmicro.2023.110118. Epub 2023 Feb 11. Int J Food Microbiol. 2023. PMID: 36796164
-
Functional microorganisms in Baijiu Daqu: Research progress and fortification strategy for application.Front Microbiol. 2023 Jan 27;14:1119675. doi: 10.3389/fmicb.2023.1119675. eCollection 2023. Front Microbiol. 2023. PMID: 36778882 Free PMC article. Review.
-
Effect of Fermentation Processing on the Flavor of Baijiu.J Agric Food Chem. 2018 Jun 6;66(22):5425-5432. doi: 10.1021/acs.jafc.8b00692. Epub 2018 May 22. J Agric Food Chem. 2018. PMID: 29751730 Review.
Cited by
-
Comparative study on physicochemical properties, microbial composition, and the volatile component of different light flavor Daqu.Food Sci Nutr. 2023 Jun 12;11(9):5174-5187. doi: 10.1002/fsn3.3476. eCollection 2023 Sep. Food Sci Nutr. 2023. PMID: 37701186 Free PMC article.
-
Pediococcus spp.-mediated competition interaction within Daqu microbiota determines the temperature formation and metabolic profiles.Appl Environ Microbiol. 2024 Apr 17;90(4):e0179023. doi: 10.1128/aem.01790-23. Epub 2024 Mar 20. Appl Environ Microbiol. 2024. PMID: 38506521 Free PMC article.
-
Evaluation of microbial communities of Chinese Feng-flavor Daqu with effects of environmental factors using traceability analysis.Sci Rep. 2023 May 11;13(1):7657. doi: 10.1038/s41598-023-34506-z. Sci Rep. 2023. PMID: 37169808 Free PMC article.
-
Microbial Diversity and Flavor Regularity of Soy Milk Fermented Using Kombucha.Foods. 2023 Feb 18;12(4):884. doi: 10.3390/foods12040884. Foods. 2023. PMID: 36832959 Free PMC article.
-
Correlation: Between Autochthonous Microbial Diversity and Volatile Metabolites During the Fermentation of Nongxiang Daqu.Front Microbiol. 2021 Sep 22;12:688981. doi: 10.3389/fmicb.2021.688981. eCollection 2021. Front Microbiol. 2021. PMID: 34630343 Free PMC article.
References
-
- Zheng XW, Tabrizi MR, Robert Nout MJ, Han BZ. 2011. Daqu—a traditional Chinese liquor fermentation starter. J Inst Brewing 117:82–90. doi:10.1002/j.2050-0416.2011.tb00447.x. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

