Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure
- PMID: 28970239
- PMCID: PMC5679176
- DOI: 10.1084/jem.20161791
Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure
Abstract
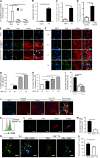
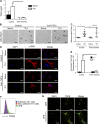
Despite emerging data indicating a role for T cells in profibrotic cardiac repair and healing after ischemia, little is known about whether T cells directly impact cardiac fibroblasts (CFBs) to promote cardiac fibrosis (CF) in nonischemic heart failure (HF). Recently, we reported increased T cell infiltration in the fibrotic myocardium of nonischemic HF patients, as well as the protection from CF and HF in TCR-α-/- mice. Here, we report that T cells activated in such a context are mainly IFN-γ+, adhere to CFB, and induce their transition into myofibroblasts. Th1 effector cells selectively drive CF both in vitro and in vivo, whereas adoptive transfer of Th1 cells, opposite to activated IFN-γ-/- Th cells, partially reconstituted CF and HF in TCR-α-/- recipient mice. Mechanistically, Th1 cells use integrin α4 to adhere to and induce TGF-β in CFB in an IFN-γ-dependent manner. Our findings identify a previously unrecognized role for Th1 cells as integrators of perivascular CF and cardiac dysfunction in nonischemic HF.
© 2017 Nevers et al.
Figures





References
-
- Blanton R.M., Takimoto E., Lane A.M., Aronovitz M., Piotrowski R., Karas R.H., Kass D.A., and Mendelsohn M.E.. 2012. Protein kinase g iα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J. Am. Heart Assoc. 1:e003731 10.1161/JAHA.112.003731 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

