Model of Chronic Equine Endometritis Involving a Pseudomonas aeruginosa Biofilm
- PMID: 28970274
- PMCID: PMC5695105
- DOI: 10.1128/IAI.00332-17
Model of Chronic Equine Endometritis Involving a Pseudomonas aeruginosa Biofilm
Abstract
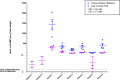
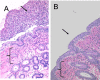
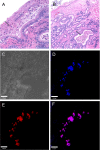
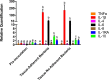
Bacteria in a biofilm community have increased tolerance to antimicrobial therapy. To characterize the role of biofilms in equine endometritis, six mares were inoculated with lux-engineered Pseudomonas aeruginosa strains isolated from equine uterine infections. Following establishment of infection, the horses were euthanized and the endometrial surfaces were imaged for luminescence to localize adherent lux-labeled bacteria. Samples from the endometrium were collected for cytology, histopathology, carbohydrate analysis, and expression of inflammatory cytokine genes. Tissue-adherent bacteria were present in focal areas between endometrial folds (6/6 mares). The Pel exopolysaccharide (biofilm matrix component) and cyclic di-GMP (biofilm-regulatory molecule) were detected in 6/6 mares and 5/6 mares, respectively, from endometrial samples with tissue-adherent bacteria (P < 0.05). A greater incidence (P < 0.05) of Pel exopolysaccharide was present in samples fixed with Bouin's solution (18/18) than in buffered formalin (0/18), indicating that Bouin's solution is more appropriate for detecting bacteria adherent to the endometrium. There were no differences (P > 0.05) in the number of inflammatory cells in the endometrium between areas with and without tissue-adherent bacteria. Neutrophils were decreased (P < 0.05) in areas surrounding tissue-adherent bacteria compared to those in areas free of adherent bacteria. Gene expression of interleukin-10, an immune-modulatory cytokine, was significantly (P < 0.05) increased in areas of tissue-adherent bacteria compared to that in endometrium absent of biofilm. These findings indicate that P. aeruginosa produces a biofilm in the uterus and that the host immune response is modulated focally around areas with biofilm, but inflammation within the tissue is similar in areas with and without biofilm matrix. Future studies will focus on therapeutic options for elimination of bacterial biofilm in the equine uterus.
Keywords: bacteria; biofilm; cyclic di-GMP; endometritis; equine; exopolysaccharide.
Copyright © 2017 Ferris et al.
Figures


Similar articles
-
In Vitro Efficacy of Nonantibiotic Treatments on Biofilm Disruption of Gram-Negative Pathogens and an In Vivo Model of Infectious Endometritis Utilizing Isolates from the Equine Uterus.J Clin Microbiol. 2016 Mar;54(3):631-9. doi: 10.1128/JCM.02861-15. Epub 2015 Dec 30. J Clin Microbiol. 2016. PMID: 26719448 Free PMC article.
-
Post-breeding inflammation and endometrial cytology in mares.Theriogenology. 2005 Aug;64(3):580-8. doi: 10.1016/j.theriogenology.2005.05.041. Theriogenology. 2005. PMID: 15978660
-
Microbiology of equine wounds and evidence of bacterial biofilms.Vet Microbiol. 2011 May 12;150(1-2):152-9. doi: 10.1016/j.vetmic.2011.01.003. Epub 2011 Jan 11. Vet Microbiol. 2011. PMID: 21273008
-
Evaluation of diagnostic methods in equine endometritis.Reprod Biol. 2016 Sep;16(3):189-196. doi: 10.1016/j.repbio.2016.06.002. Epub 2016 Jun 29. Reprod Biol. 2016. PMID: 27692361 Review.
-
Inflammatory mechanisms of endometritis.Equine Vet J. 2015 Jul;47(4):384-9. doi: 10.1111/evj.12403. Epub 2015 Apr 3. Equine Vet J. 2015. PMID: 25537084 Review.
Cited by
-
Construction of a model of endometritis in domestic rabbits using equine-derived pathogens and evaluation of therapeutic effect of sensitive drugs.Front Vet Sci. 2023 Feb 9;10:1064522. doi: 10.3389/fvets.2023.1064522. eCollection 2023. Front Vet Sci. 2023. PMID: 36846263 Free PMC article.
-
The healthy equine uterus harbors a distinct core microbiome plus a rich and diverse microbiome that varies with geographical location.Sci Rep. 2022 Aug 30;12(1):14790. doi: 10.1038/s41598-022-18971-6. Sci Rep. 2022. PMID: 36042332 Free PMC article.
-
TREM-1 deficiency attenuates the inflammatory responses in LPS-induced murine endometritis.Microb Biotechnol. 2019 Nov;12(6):1337-1345. doi: 10.1111/1751-7915.13467. Epub 2019 Jul 31. Microb Biotechnol. 2019. PMID: 31365951 Free PMC article.
-
Isolation of lactic acid bacteria from the reproductive tract of mares as potentially beneficial strains to prevent equine endometritis.Vet Res Commun. 2024 Jun;48(3):1353-1366. doi: 10.1007/s11259-024-10295-2. Epub 2024 Jan 18. Vet Res Commun. 2024. PMID: 38233700
-
Whole Genome Sequencing and CRISPR/Cas9 Gene Editing of Enterotoxigenic Escherichia coli BE311 for Fluorescence Labeling and Enterotoxin Analyses.Int J Mol Sci. 2022 Jul 6;23(14):7502. doi: 10.3390/ijms23147502. Int J Mol Sci. 2022. PMID: 35886856 Free PMC article.
References
-
- Traub-Dargatz JL, Salman MD, Voss JL. 1991. Medical problems of adult horses, as ranked by equine practitioners. J Am Vet Med Assoc 198:1745–1747. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

