Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors
- PMID: 28970469
- PMCID: PMC5624946
- DOI: 10.1038/s41467-017-00716-z
Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors
Abstract
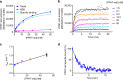
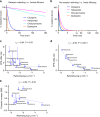
Atypical antipsychotic drugs (APDs) have been hypothesized to show reduced extrapyramidal side effects (EPS) due to their rapid dissociation from the dopamine D2 receptor. However, support for this hypothesis is limited to a relatively small number of observations made across several decades and under different experimental conditions. Here we show that association rates, but not dissociation rates, correlate with EPS. We measured the kinetic binding properties of a series of typical and atypical APDs in a novel time-resolved fluorescence resonance energy transfer assay, and correlated these properties with their EPS and prolactin-elevating liabilities at therapeutic doses. EPS are robustly predicted by a rebinding model that considers the microenvironment of postsynaptic D2 receptors and integrates association and dissociation rates to calculate the net rate of reversal of receptor blockade. Thus, optimizing binding kinetics at the D2 receptor may result in APDs with improved therapeutic profile.Atypical antipsychotics show reduced extrapyramidal side effects compared to first generation drugs. Here the authors use time-resolved FRET to measure binding kinetics, and show that side effects correlate with drug association rates to the D2 receptor, while dissociation rates correlate with prolactin elevation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Comment in
-
Antipsychotics with similar association kinetics at dopamine D2 receptors differ in extrapyramidal side-effects.Nat Commun. 2018 Sep 3;9(1):3577. doi: 10.1038/s41467-018-04489-x. Nat Commun. 2018. PMID: 30177830 Free PMC article. No abstract available.
References
-
- Farde L, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch. Gen. Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

