Mast Cell-Intervertebral disc cell interactions regulate inflammation, catabolism and angiogenesis in Discogenic Back Pain
- PMID: 28970490
- PMCID: PMC5624870
- DOI: 10.1038/s41598-017-12666-z
Mast Cell-Intervertebral disc cell interactions regulate inflammation, catabolism and angiogenesis in Discogenic Back Pain
Abstract
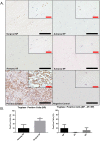
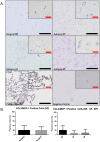
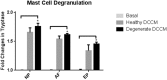
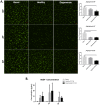
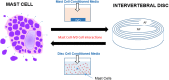
Low back pain (LBP) is a widespread debilitating disorder of significant socio-economic importance and intervertebral disc (IVD) degeneration has been implicated in its pathogenesis. Despite its high prevalence the underlying causes of LBP and IVD degeneration are not well understood. Recent work in musculoskeletal degenerative diseases such as osteoarthritis have revealed a critical role for immune cells, specifically mast cells in their pathophysiology, eluding to a potential role for these cells in the pathogenesis of IVD degeneration. This study sought to characterize the presence and role of mast cells within the IVD, specifically, mast cell-IVD cell interactions using immunohistochemistry and 3D in-vitro cell culture methods. Mast cells were upregulated in painful human IVD tissue and induced an inflammatory, catabolic and pro-angiogenic phenotype in bovine nucleus pulposus and cartilage endplate cells at the gene level. Healthy bovine annulus fibrosus cells, however, demonstrated a protective role against key inflammatory (IL-1β and TNFα) and pro-angiogenic (VEGFA) genes expressed by mast cells, and mitigated neo-angiogenesis formation in vitro. In conclusion, mast cells can infiltrate and elicit a degenerate phenotype in IVD cells, enhancing key disease processes that characterize the degenerate IVD, making them a potential therapeutic target for LBP.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

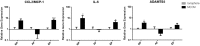


References
-
- Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J. Bone Joint Surg. Am. 2006;88(Suppl 2):21–24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

