Autosomal dominant transmission of complicated hereditary spastic paraplegia due to a dominant negative mutation of KIF1A, SPG30 gene
- PMID: 28970574
- PMCID: PMC5624960
- DOI: 10.1038/s41598-017-12999-9
Autosomal dominant transmission of complicated hereditary spastic paraplegia due to a dominant negative mutation of KIF1A, SPG30 gene
Abstract
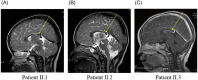
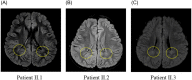
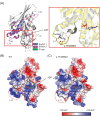
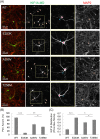
KIF1A is a brain-specific anterograde motor protein that transports cargoes towards the plus-ends of microtubules. Many variants of the KIF1A gene have been associated with neurodegenerative diseases and developmental delay. Homozygous mutations of KIF1A have been identified in a recessive subtype of hereditary spastic paraplegia (HSP), SPG30. In addition, KIF1A mutations have been found in pure HSP with autosomal dominant inheritance. Here we report the first case of familial complicated HSP with a KIF1A mutation transmitted in autosomal dominant inheritance. A heterozygous p.T258M mutation in KIF1A was found in a Korean family through targeted exome sequencing. They displayed phenotypes of mild intellectual disability with language delay, epilepsy, optic nerve atrophy, thinning of corpus callosum, periventricular white matter lesion, and microcephaly. A structural modeling revealed that the p.T258M mutation disrupted the binding of KIF1A motor domain to microtubules and its movement along microtubules. Assays of peripheral accumulation and proximal distribution of KIF1A motor indicated that the KIF1A motor domain with p.T258M mutation has reduced motor activity and exerts a dominant negative effect on wild-type KIF1A. These results suggest that the p.T258M mutation suppresses KIF1A motor activity and induces complicated HSP accompanying intellectual disability transmitted in autosomal dominant inheritance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

