Construction of nanostructures for selective lithium ion conduction using self-assembled molecular arrays in supramolecular solids
- PMID: 28970871
- PMCID: PMC5613908
- DOI: 10.1080/14686996.2017.1366816
Construction of nanostructures for selective lithium ion conduction using self-assembled molecular arrays in supramolecular solids
Abstract
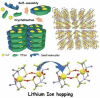
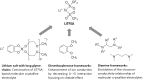
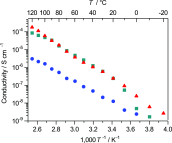
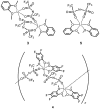
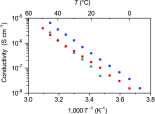
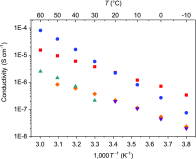
In the development of innovative molecule-based materials, the identification of the structural features in supramolecular solids and the understanding of the correlation between structure and function are important factors. The author investigated the development of supramolecular solid electrolytes by constructing ion conduction paths using a supramolecular hierarchical structure in molecular crystals because the ion conduction path is an attractive key structure due to its ability to generate solid-state ion diffusivity. The obtained molecular crystals exhibited selective lithium ion diffusion via conduction paths consisting of lithium bis(trifluoromethanesulfonyl)amide (LiTFSA) and small molecules such as ether or amine compounds. In the present review, the correlation between the crystal structure and ion conductivity of the obtained molecular crystals is addressed based on the systematic structural control of the ionic conduction paths through the modification of the component molecules. The relationship between the crystal structure and ion conductivity of the molecular crystals provides a guideline for the development of solid electrolytes based on supramolecular solids exhibiting rapid and selective lithium ion conduction.
Keywords: 101 Self-assembly / Self-organized materials; 20 Organic and soft materials (colloids, liquid crystals, gel, polymers); 206 Energy conversion / transport / storage / recovery; 501 Chemical analyses; 504 X-ray / Neutron diffraction and scattering; Ion conduction; lithium; molecular crystals; self-assembly; solid electrolytes; supramolecules.
Figures






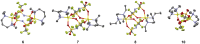

Similar articles
-
Structural design of ionic conduction paths in molecular crystals for selective and enhanced lithium ion conduction.Chemistry. 2013 Sep 27;19(40):13554-60. doi: 10.1002/chem.201300106. Epub 2013 Aug 12. Chemistry. 2013. PMID: 23939993
-
Molecular ionics in supramolecular assemblies with channel structures containing lithium ions.Chemistry. 2012 Nov 26;18(48):15305-9. doi: 10.1002/chem.201202056. Epub 2012 Nov 5. Chemistry. 2012. PMID: 23129395
-
Organic Crystalline Solid Electrolytes with High Mg-Ion Conductivity Composed of Nonflammable Ionic Liquid Analogs and Mg(TFSA)2.Inorg Chem. 2022 May 16;61(19):7358-7364. doi: 10.1021/acs.inorgchem.2c00307. Epub 2022 May 3. Inorg Chem. 2022. PMID: 35504045
-
Supramolecular Chemistry in Microflow Fields: Toward a New Material World of Precise Kinetic Control.Chem Asian J. 2015 Dec;10(12):2574-88. doi: 10.1002/asia.201500555. Epub 2015 Sep 15. Chem Asian J. 2015. PMID: 26288064 Review.
-
Review on Polymer-Based Composite Electrolytes for Lithium Batteries.Front Chem. 2019 Aug 8;7:522. doi: 10.3389/fchem.2019.00522. eCollection 2019. Front Chem. 2019. PMID: 31440498 Free PMC article. Review.
Cited by
-
Investigating the Interface of Li{N(SO2F)2}(NCCH2CH2CN)2 Molecular Crystal Electrolytes for 5 V Class Solid-State Batteries.ACS Appl Mater Interfaces. 2025 Apr 9;17(14):21951-21957. doi: 10.1021/acsami.4c22076. Epub 2025 Mar 28. ACS Appl Mater Interfaces. 2025. PMID: 40154500
References
-
- Busseron E, Ruff Y, Moulin E, et al. . Supramolecular self-assemblies as functional nanomaterials. Nanoscale. 2013;5:7098–7140. - PubMed
-
- Zhanhu S, Gilles A, Kocsis I, et al. . Squalyl crown ether self-assembled conjugates: an example of highly selective artificial K+ channels. Chem Eur J. 2016;22:2158–2164. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
