A pH responsive complexation-based drug delivery system for oxaliplatin
- PMID: 28970876
- PMCID: PMC5618340
- DOI: 10.1039/c7sc01438d
A pH responsive complexation-based drug delivery system for oxaliplatin
Abstract
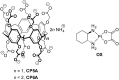
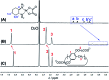
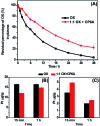
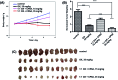
A responsive drug delivery system (DDS) for oxaliplatin (OX) has been designed with a view to overcoming several drawbacks associated with this anticancer agent, including fast degradation/deactivation in the blood stream, lack of tumor selectivity, and low bioavailability. The present approach is based on the direct host-guest encapsulation of OX by a pH-responsive receptor, carboxylatopillar[6]arene (CP6A). The binding affinities of CP6A for OX were found to be pH-sensitive at biologically relevant pH. For example, the association constant (Ka) at pH 7.4 [Ka = (1.02 ± 0.05) × 104 M-1] is 24 times larger than that at pH 5.4 [Ka = (4.21 ± 0.06) × 102 M-1]. Encapsulation of OX within the CP6A cavity did not affect its in vitro cytotoxicity as inferred from comparison studies carried out in several cancer cells (e.g., the HepG-2, MCF-7, and A549 cell lines). On the other hand, complexation by CP6A serves to increase the inherent stability of OX in plasma by 2.8-fold over a 24 h incubation period. The formation of a CP6A⊃OX host-guest complex served to enhance in a statistically significant way the ability of OX to inhibit the regrowth of sarcoma 180 (S180) tumors in Kunming (KM) mice xenografts. The improved anticancer activity observed in vivo for CP6A⊃OX is attributed to the combined effects of enhanced stability of the host-guest complex and the pH-responsive release of OX. Specifically, it is proposed that OX is protected as the result of complex formation and then released effectively in the acidic tumor environment.
Figures

Similar articles
-
Host-guest complexation of cyclophosphamide by carboxylatopillar[6]arene for increasing stability and enhancing its curative effect on breast carcinoma.Bioorg Med Chem Lett. 2022 Dec 15;78:129060. doi: 10.1016/j.bmcl.2022.129060. Epub 2022 Nov 9. Bioorg Med Chem Lett. 2022. PMID: 36371019
-
Supramolecular combination chemotherapy: a pH-responsive co-encapsulation drug delivery system.Chem Sci. 2020 Jun 3;11(24):6275-6282. doi: 10.1039/d0sc01756f. eCollection 2020 Jun 28. Chem Sci. 2020. PMID: 32953023 Free PMC article.
-
Synergistic enhancement of the emergency treatment effect of organophosphate poisoning by a supramolecular strategy.Chem Sci. 2021 Mar 1;12(14):5202-5208. doi: 10.1039/d1sc00426c. Chem Sci. 2021. PMID: 34163757 Free PMC article.
-
Host-Guest Formulation of Carboxylatopillar[6]arene with Ergothioneine as a New Antidote for Combinational Detoxification of Paraquat.ACS Appl Mater Interfaces. 2023 Jun 7;15(22):26407-26416. doi: 10.1021/acsami.3c03259. Epub 2023 May 23. ACS Appl Mater Interfaces. 2023. PMID: 37218650
-
MicroRNAs in cancer therapy: Their involvement in oxaliplatin sensitivity/resistance of cancer cells with a focus on colorectal cancer.Life Sci. 2020 Sep 1;256:117973. doi: 10.1016/j.lfs.2020.117973. Epub 2020 Jun 20. Life Sci. 2020. PMID: 32569779 Review.
Cited by
-
Separation of pyrrolidine from tetrahydrofuran by using pillar[6]arene-based nonporous adaptive crystals.Chem Sci. 2022 Jun 2;13(25):7536-7540. doi: 10.1039/d2sc02494b. eCollection 2022 Jun 29. Chem Sci. 2022. PMID: 35872814 Free PMC article.
-
Platinum(IV)-Ferrocene Conjugates and Their Cyclodextrin Host-Guest Complexes.Inorg Chem. 2019 Jun 17;58(12):7886-7894. doi: 10.1021/acs.inorgchem.9b00570. Epub 2019 May 24. Inorg Chem. 2019. PMID: 31125214 Free PMC article.
-
Multi-spectroscopic monitoring of molecular interactions between an amino acid-functionalized ionic liquid and potential anti-Alzheimer's drugs.RSC Adv. 2020 Oct 23;10(64):38873-38883. doi: 10.1039/d0ra06323a. eCollection 2020 Oct 21. RSC Adv. 2020. PMID: 35518436 Free PMC article.
-
Recent developments on zinc(ii) metal-organic framework nanocarriers for physiological pH-responsive drug delivery.Medchemcomm. 2019 Oct 17;10(12):2038-2051. doi: 10.1039/c9md00400a. eCollection 2019 Dec 1. Medchemcomm. 2019. PMID: 32206240 Free PMC article. Review.
-
Use of Half-Generation PAMAM Dendrimers (G0.5-G3.5) with Carboxylate End-Groups to Improve the DACHPtCl2 and 5-FU Efficacy as Anticancer Drugs.Molecules. 2021 May 14;26(10):2924. doi: 10.3390/molecules26102924. Molecules. 2021. PMID: 34069054 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

