Structural-functional analysis of engineered protein-nanoparticle assemblies using graphene microelectrodes
- PMID: 28970912
- PMCID: PMC5607901
- DOI: 10.1039/c7sc01565h
Structural-functional analysis of engineered protein-nanoparticle assemblies using graphene microelectrodes
Abstract
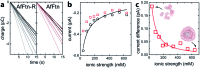
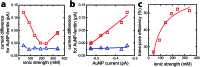
The characterization of protein-nanoparticle assemblies in solution remains a challenge. We demonstrate a technique based on a graphene microelectrode for structural-functional analysis of model systems composed of nanoparticles enclosed in open-pore and closed-pore ferritin molecules. The method readily resolves the difference in accessibility of the enclosed nanoparticle for charge transfer and offers the prospect for quantitative analysis of pore-mediated transport, while shedding light on the spatial orientation of the protein subunits on the nanoparticle surface, faster and with higher sensitivity than conventional catalysis methods.
Figures


Similar articles
-
Electromagnetic Field Redistribution in Metal Nanoparticle on Graphene.Nanoscale Res Lett. 2018 Apr 25;13(1):124. doi: 10.1186/s11671-018-2535-0. Nanoscale Res Lett. 2018. PMID: 29696469 Free PMC article.
-
Modular Homogeneous Chromophore-Catalyst Assemblies.Acc Chem Res. 2016 May 17;49(5):835-43. doi: 10.1021/acs.accounts.5b00539. Epub 2016 Apr 22. Acc Chem Res. 2016. PMID: 27104312
-
Detection of Dopamine Based on Aptamer-Modified Graphene Microelectrode.Sensors (Basel). 2024 May 5;24(9):2934. doi: 10.3390/s24092934. Sensors (Basel). 2024. PMID: 38733043 Free PMC article.
-
Recent Advances in Metallic Nanoparticle Assemblies for Surface-Enhanced Spectroscopy.Int J Mol Sci. 2021 Dec 28;23(1):291. doi: 10.3390/ijms23010291. Int J Mol Sci. 2021. PMID: 35008714 Free PMC article. Review.
-
Distance and orientation dependence of excitation energy transfer: from molecular systems to metal nanoparticles.J Phys Chem B. 2009 Feb 19;113(7):1817-32. doi: 10.1021/jp806536w. J Phys Chem B. 2009. PMID: 19128043 Review.
Cited by
-
All-Electronic Quantification of Neuropeptide-Receptor Interaction Using a Bias-Free Functionalized Graphene Microelectrode.ACS Nano. 2018 May 22;12(5):4218-4223. doi: 10.1021/acsnano.7b07474. Epub 2018 Apr 17. ACS Nano. 2018. PMID: 29634231 Free PMC article.
-
Ferritin-based drug delivery systems: Hybrid nanocarriers for vascular immunotargeting.J Control Release. 2018 Jul 28;282:13-24. doi: 10.1016/j.jconrel.2018.02.042. Epub 2018 Mar 6. J Control Release. 2018. PMID: 29522833 Free PMC article. Review.
-
A protein-protein host-guest complex: Thermostable ferritin encapsulating positively supercharged green fluorescent protein.Protein Sci. 2018 Oct;27(10):1755-1766. doi: 10.1002/pro.3483. Protein Sci. 2018. PMID: 30051936 Free PMC article.
-
Exchange Coupling Effects on the Magnetotransport Properties of Ni-Nanoparticle-Decorated Graphene.Nanomaterials (Basel). 2023 Jun 15;13(12):1861. doi: 10.3390/nano13121861. Nanomaterials (Basel). 2023. PMID: 37368291 Free PMC article.
References
-
- Fantechi E., Innocenti C., Zanardelli M., Fittipaldi M., Falvo E., Carbo M., Shullani V., Mannelli L. D. C., Ghelardini C., Ferretti A. M., Ponti A., Sangregorio C., Ceci P. ACS Nano. 2014;8:4705–4719. - PubMed
-
- Kumar S., Aaron J., Sokolov K. Nat. Protoc. 2008;3:314–320. - PubMed
-
- Medintz I. L., Uyeda H. T., Goldman E. R., Mattoussi H. Nat. Mater. 2005;4:435–446. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

