Chemical reaction within a compact non-porous crystal containing molecular clusters without the loss of crystallinity
- PMID: 28970914
- PMCID: PMC5609145
- DOI: 10.1039/c7sc01041a
Chemical reaction within a compact non-porous crystal containing molecular clusters without the loss of crystallinity
Abstract
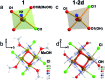
The very rare occurrence of a gas-solid chemical reaction has been found to take place on a molecule within a compact non-porous crystal without destroying its long-range structural order and retaining similar crystal structures when yellow crystals of FeII4(mbm)4Cl4(MeOH)4 were exposed to air to give black [FeIII4(mbm)4Cl4(OH)4]·2H2O. The latter cannot be synthesised directly. The original cluster underwent an exchange of methanol to hydroxide, an oxidation of Fe(ii) to Fe(iii), a change in stereochemistry and hydration while the packing and space-group remained unaltered.
Figures



References
-
- Marin G. and Yablonsky G. S., Kinetics of Chemical Reactions, Wiley-VCH, Weinheim, 2011.
-
- Cheetham A. K. and Day P., Solid state chemistry techniques, Clarendon Press, Lincoln, United Kingdom, 1990.
-
- Papaefstathiou G. S., Zhong Z., Geng L., MacGillivray L. R. J. Am. Chem. Soc. 2004;126:9158–9159. - PubMed
-
- Three special issues for metal–organic framework reviews. Chem. Soc. Rev., 2009, 38, 1201 1508 Chem. Rev., 2012, 112, 673, 1268 Chem. Soc. Rev., 2014, 43, 5403 6176.
-
- Kawano M., Fujita M. Coord. Chem. Rev. 2007;251:2592–2605.
LinkOut - more resources
Full Text Sources
Other Literature Sources

