IVIG-Associated Maternal Pancytopenia during Treatment for Neonatal Alloimmune Thrombocytopenia
- PMID: 28970962
- PMCID: PMC5621970
- DOI: 10.1055/s-0037-1607055
IVIG-Associated Maternal Pancytopenia during Treatment for Neonatal Alloimmune Thrombocytopenia
Abstract
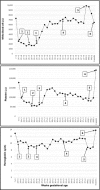
Background Treatment for neonatal alloimmune thrombocytopenia (NAIT) primarily involves maternal administration of intravenous immunoglobulin (IVIG) therapy and prednisone according to protocols based on risk stratification. While IVIG is generally well tolerated, hematologic side effects are a potential complication. Case We present the successful management of a rare complication of maternal pancytopenia following standard IVIG treatment. Diagnosis was made during routine obstetric exams. Management included reducing IVIG dosage and adding daily prednisone. Additionally, infusion Lots possibly associated with the event were identified and avoided. Interventions resulted in the resolution of pancytopenia and the birth of a healthy infant without thrombocytopenia. Conclusion Pancytopenia is a rare complication of IVIG treatment in women with pregnancies complicated by NAIT. Serial complete blood counts at the time of treatment would allow for early detection and timely management of the patient. Additionally, limiting the number of infusion Lots may decrease the chance of the described complications.
Keywords: IVIG; NAIT; blood count; pancytopenia; prednisone.
Figures
References
-
- Berkowitz R L, Bussel J B, McFarland J G. Alloimmune thrombocytopenia: state of the art 2006. Am J Obstet Gynecol. 2006;195(04):907–913. - PubMed
-
- Berkowitz R L, Lesser M L, McFarland J Get al. Antepartum treatment without early cordocentesis for standard-risk alloimmune thrombocytopenia: a randomized controlled trial Obstet Gynecol 2007110(2 Pt 1):249–255. - PubMed
-
- Pacheco L D, Berkowitz R L, Moise K J, Jr, Bussel J B, McFarland J G, Saade G R. Fetal and neonatal alloimmune thrombocytopenia: a management algorithm based on risk stratification. Obstet Gynecol. 2011;118(05):1157–1163. - PubMed
-
- Rink B D, Gonik B, Chmait R H, O'Shaughnessy R.Maternal hemolysis after intravenous immunoglobulin treatment in fetal and neonatal alloimmune thrombocytopenia Obstet Gynecol 2013121(2 Pt 2, Suppl 1)471–473. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

