Role of the guanine nucleotide binding protein, Gαo, in the development of morphine tolerance and dependence
- PMID: 28971229
- PMCID: PMC5819733
- DOI: 10.1007/s00213-017-4742-2
Role of the guanine nucleotide binding protein, Gαo, in the development of morphine tolerance and dependence
Abstract
Rationale: The use of morphine and other opioids for chronic pain is limited by the development of analgesic tolerance and physical dependence. Morphine produces its effects by activating the μ opioid receptor, which couples to Gαi/o-containing heterotrimeric G proteins. Evidence suggests that the antinociceptive effects of morphine are mediated by Gαo. However, the role of Gαo in the development of morphine tolerance and dependence is unknown.
Objective: The objective of the study is to evaluate the contribution of Gαo to the development of morphine tolerance and dependence in mice.
Methods: 129S6 mice lacking one copy of the Gαo gene (Gαo +/-) were administered morphine acutely or chronically. Mice were examined for tolerance to the antinociceptive action of morphine using the 52 °C hot plate as the nociceptive stimulus and for dependence by evaluating the severity of naltrexone-precipitated withdrawal. Wild-type littermates of the Gαo +/- mice were used as controls. Changes in μ receptor number and function were determined in midbrain and hindbrain homogenates using radioligand binding and μ agonist-stimulated [35S]GTPγS binding, respectively.
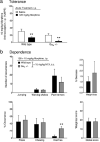
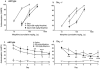
Results: Following either acute or chronic morphine treatment, all mice developed antinociceptive tolerance and physical dependence, regardless of genotype. With chronic morphine treatment, Gαo +/- mice developed tolerance faster and displayed more severe naltrexone-precipitated withdrawal in some behaviors than did wild-type littermates. Morphine tolerance was not associated with changes in μ receptor number or function in brain homogenates from either wild-type or Gαo +/- mice.
Conclusions: These data suggest that the guanine nucleotide binding protein Gαo offers some protection against the development of morphine tolerance and dependence.
Keywords: Antinociception; G protein; Morphine; Receptor binding; Tolerance; Withdrawal; μ receptor.
Conflict of interest statement
Figures


References
-
- Bagley EE, Gerke MB, Vaughan CW, Hack SP, Christie MJ. GABA transporter currents activated by protein kinase A excite midbrain neurons during opioid withdrawal. Neuron. 2005b;45:433–445. - PubMed
-
- Buntin-Mushock C, Phillip L, Moriyama K, Palmer PP. Age-dependent opioid escalation in chronic pain patients. Anesth Analg. 2005;100:1740–1745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

