Large cosolutes, small cosolutes, and dihydrofolate reductase activity
- PMID: 28971539
- PMCID: PMC5699487
- DOI: 10.1002/pro.3316
Large cosolutes, small cosolutes, and dihydrofolate reductase activity
Erratum in
-
CORRECTION.Protein Sci. 2019 Sep;28(9):1744. doi: 10.1002/pro.3697. Protein Sci. 2019. PMID: 31424142 Free PMC article. No abstract available.
Abstract
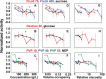
Protein enzymes are the main catalysts in the crowded and complex cellular interior, but their activity is almost always studied in dilute buffered solutions. Studies that attempt to recreate the cellular interior in vitro often utilize synthetic polymers as crowding agents. Here, we report the effects of the synthetic polymer cosolutes Ficoll, dextran, and polyvinylpyrrolidone, and their respective monomers, sucrose, glucose, and 1-ethyl-2-pyrrolidone, on the activity of the 18-kDa monomeric enzyme, Escherichia coli dihydrofolate reductase. At low concentrations, reductase activity increases relative to buffer and monomers, suggesting a macromolecular effect. However, the effect decreases at higher concentrations, approaching, and, in some cases, falling below buffer values. We also assessed activity in terms of volume occupancy, viscosity, and the overlap concentration (where polymers form an interwoven mesh). The trends vary with polymer family, but changes in activity are within threefold of buffer values. We also compiled and analyzed results from previous studies and conclude that alterations of steady-state enzyme kinetics in solutions crowded with synthetic polymers are idiosyncratic with respect to the crowding agent and enzyme.
Keywords: concentration-dependent crowding; crowding and enzyme activity; enzyme kinetics; macromolecular crowding; size-dependent crowding.
© 2017 The Protein Society.
Figures


Similar articles
-
Crowders Steal Dihydrofolate Reductase Ligands through Quinary Interactions.Biochemistry. 2019 Mar 5;58(9):1198-1213. doi: 10.1021/acs.biochem.8b01110. Epub 2019 Feb 18. Biochemistry. 2019. PMID: 30724552 Free PMC article.
-
Does R67 dihydrofolate reductase possess a proton donor?Adv Exp Med Biol. 1993;338:493-8. doi: 10.1007/978-1-4615-2960-6_99. Adv Exp Med Biol. 1993. PMID: 8304165 No abstract available.
-
In Vivo Titration of Folate Pathway Enzymes.Appl Environ Microbiol. 2018 Sep 17;84(19):e01139-18. doi: 10.1128/AEM.01139-18. Print 2018 Oct 1. Appl Environ Microbiol. 2018. PMID: 30030232 Free PMC article.
-
Macromolecular Crowding Is More than Hard-Core Repulsions.Annu Rev Biophys. 2022 May 9;51:267-300. doi: 10.1146/annurev-biophys-091321-071829. Epub 2022 Mar 3. Annu Rev Biophys. 2022. PMID: 35239418 Review.
-
Free-energy landscape of enzyme catalysis.Biochemistry. 2008 Mar 18;47(11):3317-21. doi: 10.1021/bi800049z. Epub 2008 Feb 26. Biochemistry. 2008. PMID: 18298083 Review.
Cited by
-
An in vitro mimic of in-cell solvation for protein folding studies.Protein Sci. 2020 Apr;29(4):1060-1068. doi: 10.1002/pro.3833. Epub 2020 Feb 6. Protein Sci. 2020. PMID: 31994240 Free PMC article.
-
Crowders Steal Dihydrofolate Reductase Ligands through Quinary Interactions.Biochemistry. 2019 Mar 5;58(9):1198-1213. doi: 10.1021/acs.biochem.8b01110. Epub 2019 Feb 18. Biochemistry. 2019. PMID: 30724552 Free PMC article.
-
Cell stress and phase separation stabilize the monomeric state of pseudoisocyanine chloride employed as a self-assembly crowding sensor.Commun Chem. 2024 Oct 7;7(1):230. doi: 10.1038/s42004-024-01315-y. Commun Chem. 2024. PMID: 39375435 Free PMC article.
-
Glucose Oxidase Immobilized on Magnetic Zirconia: Controlling Catalytic Performance and Stability.ACS Omega. 2020 May 20;5(21):12329-12338. doi: 10.1021/acsomega.0c01067. eCollection 2020 Jun 2. ACS Omega. 2020. PMID: 32548416 Free PMC article.
-
Using NMR-detected hydrogen-deuterium exchange to quantify protein stability in cosolutes, under crowded conditions in vitro and in cells.Magn Reson Lett. 2023 May 5;3(4):319-326. doi: 10.1016/j.mrl.2023.04.003. eCollection 2023 Nov. Magn Reson Lett. 2023. PMID: 40919504 Free PMC article. Review.
References
-
- Zeskind BJ, Jordan CD, Timp W, Trapani L, Waller G, Horodincu V, Erhrlich DJ, Matsudaira P (2007) Nucleic acid and protein mass mapping by live‐cell deep‐ultraviolet microscopy. Nat Methods 4:567–569. - PubMed
-
- Cheung MC, LaCroix R, McKenna BK, Liu L, Winkelman J, Ehrlich DJ (2013) Intracellular protein and nucleic acid measured in eight cell types using deep‐ultraviolet mass mapping. Cytometry A 83:540–551. - PubMed
-
- Zimmerman S, Trach S (1991) Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli . J Mol Biol 222:599–620. - PubMed
-
- Cayley S, Lewis BA, Guttman HJ, Record MT (1991) Characterization of the cytoplasm of Escherichia coli K‐12 as a function of external osmolarity: implications for protein‐DNA interactions in vivo . J Mol Biol 2:281–300. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

