Dietary N-Glycans from Bovine Lactoferrin and TLR Modulation
- PMID: 28971586
- PMCID: PMC6120133
- DOI: 10.1002/mnfr.201700389
Dietary N-Glycans from Bovine Lactoferrin and TLR Modulation
Abstract
Scope: Bovine lactoferrin (bLF) is an ingredient of food supplements and infant formulas given its antimicrobial and antiviral properties. We modified bLF enzymatically to alter its N-glycosylation and to isolate the glycan chains. The aims of this study include (1) to evaluate whether such derivates induce responses via pattern recognition receptors namely Toll-like receptors (TLRs) and (2) to relate those responses to their different glycosylation profiles.
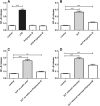
Methods and results: The unmodified and modified bLF fractions are incubated with reporter cell lines expressing pattern recognition receptors. Afterwards, we screen for TLRs and analyze for nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) activation. Activation of reporter cell lines show that signaling is highly dependent on TLRs. The activation pattern of bLF is reduced with the desialylated form and increased with the demannosylated form. In reporter cells for TLR, bLF activate TLR-4 and inhibit TLR-3. The isolated glycans from bLF inhibit TLR-8. TLR-2, TLR-5, TLR-7, and TLR-9 are not significantly altered.
Conclusion: The profile of glycosylation is key for the biological activity of bLF. By understanding how this affects the human defense responses, the bLF glycan profile can be modified to enhance its immunomodulatory effects when used as a dietary ingredient.
Keywords: N-glycosylation; NF-κB; bovine lactoferrin; toll-like receptors.
© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






References
-
- Satue‐Gracia M. T., Frankel E. N., Rangavajhyala N., German J. B., J. Agric. Food Chem. 2000, 48, 4984. - PubMed
-
- Aly E., Ros G., Frontela C., J. Food Res. 2013, 2, 25.
-
- Buccigrossi V., De Marco G., Bruzzese E., Ombrato L., Bracale I., Polito G., Guarino A., Pediatr. Res. 2007, 61, 410. - PubMed
-
- Liao Y. L., Jiang R. L., Lonnerdal B., Biochem. Cell Biol. Biol. Cell. 2012, 90, 476. - PubMed
-
- Steijns J. M., Int. J. Dairy Technol. 2001, 54, 81.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

