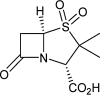
Kinetics of Sulbactam Hydrolysis by β-Lactamases, and Kinetics of β-Lactamase Inhibition by Sulbactam
- PMID: 28971872
- PMCID: PMC5700308
- DOI: 10.1128/AAC.01612-17
Kinetics of Sulbactam Hydrolysis by β-Lactamases, and Kinetics of β-Lactamase Inhibition by Sulbactam
Abstract
Sulbactam is one of four β-lactamase inhibitors in current clinical use to counteract drug resistance caused by degradation of β-lactam antibiotics by these bacterial enzymes. As a β-lactam itself, sulbactam is susceptible to degradation by β-lactamases. I investigated the Michaelis-Menten kinetics of sulbactam hydrolysis by 14 β-lactamases, representing clinically widespread groups within all four Ambler classes, i.e., CTX-M-15, KPC-2, SHV-5, and TEM-1 for class A; IMP-1, NDM-1, and VIM-1 for class B; Acinetobacter baumannii ADC-7, Pseudomonas aeruginosa AmpC, and Enterobacter cloacae P99 for class C; and OXA-10, OXA-23, OXA-24, and OXA-48 for class D. All of the β-lactamases were able to hydrolyze sulbactam, although they varied widely in their kinetic constants for the reaction, even within each class. I also investigated the inactivation kinetics of the inhibition of these enzymes by sulbactam. The class A β-lactamases varied widely in their susceptibility to inhibition, the class C and D enzymes were very weakly inhibited, and the class B enzymes were essentially or completely unaffected. In addition, we measured the sulbactam turnover number, the sulbactam/enzyme molar ratio required for complete inhibition of each enzyme. Class C enzymes had the lowest turnover numbers, class A enzymes varied widely, and class D enzymes had very high turnover numbers. These results are valuable for understanding which β-lactamases ought to be well inhibited by sulbactam. Moreover, since sulbactam has intrinsic antibacterial activity against Acinetobacter species pathogens, these results contribute to understanding β-lactamase-mediated sulbactam resistance in Acinetobacter, especially due to the action of the widespread class D enzymes.
Keywords: kinetics; sulbactam; turnover number; β-lactamase.
Copyright © 2017 American Society for Microbiology.
References
-
- Castanheira M, Farrell SE, Deshpande LM, Mendes RE, Jones RN. 2013. Prevalence of β-lactamase-encoding genes among Enterobacteriaceae bacteremia isolates collected in 26 U.S. hospitals: report from the SENTRY antimicrobial surveillance program (2010). Antimicrob Agents Chemother 57:3012–3020. doi: 10.1128/AAC.02252-12. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous