DBZ (Danshensu Bingpian Zhi), a Novel Natural Compound Derivative, Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice
- PMID: 28971954
- PMCID: PMC5721843
- DOI: 10.1161/JAHA.117.006297
DBZ (Danshensu Bingpian Zhi), a Novel Natural Compound Derivative, Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice
Abstract
Background: DBZ (Danshensu Bingpian Zhi), a synthetic derivative of a natural compound found in traditional Chinese medicine, has been reported to suppress lipopolysaccharide-induced macrophage activation and lipid accumulation in vitro. The aim of this study was to assess whether DBZ could attenuate atherosclerosis at early and advanced stages.
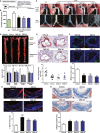
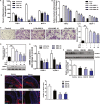
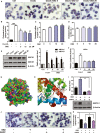
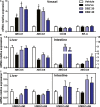
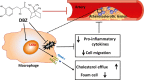
Methods and results: The effects of DBZ on the development of atherosclerosis were studied using apolipoprotein E-deficient (apoE-/-) mice. For early treatment, 5-week-old apoE-/- mice were fed a Western diet and treated daily by oral gavage with or without DBZ or atorvastatin for 10 weeks. For advanced treatment, 5-week-old apoE-/- mice were fed a Western diet for 10 weeks to induce atherosclerosis, and then they were randomly divided into 4 groups and subjected to the treatment of vehicle, 20 mg/kg per day DBZ, 40 mg/kg per day DBZ, or 10 mg/kg per day atorvastatin for the subsequent 10 weeks. We showed that early treatment of apoE-/- mice with DBZ markedly reduced atherosclerotic lesion formation by inhibiting inflammation and decreasing macrophage infiltration into the vessel wall. Treatment with DBZ also attenuated the progression of preestablished diet-induced atherosclerotic plaques in apoE-/- mice. In addition, we showed that DBZ may affect LXR (liver X receptor) function and that treatment of macrophages with DBZ suppressed lipopolysaccharide-stimulated cell migration and oxidized low-density lipoprotein-induced foam cell formation.
Conclusions: DBZ potentially has antiatherosclerotic effects that involve the inhibition of inflammation, macrophage migration, leukocyte adhesion, and foam cell formation. These results suggest that DBZ may be used as a therapeutic agent for the prevention and treatment of atherosclerosis.
Keywords: LXR; atherosclerosis; foam cell; inflammation.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures

Similar articles
-
Rosuvastatin Reduces Aortic Sinus and Coronary Artery Atherosclerosis in SR-B1 (Scavenger Receptor Class B Type 1)/ApoE (Apolipoprotein E) Double Knockout Mice Independently of Plasma Cholesterol Lowering.Arterioscler Thromb Vasc Biol. 2018 Jan;38(1):26-39. doi: 10.1161/ATVBAHA.117.305140. Epub 2017 Nov 21. Arterioscler Thromb Vasc Biol. 2018. PMID: 29162602 Free PMC article.
-
Chondroitin Sulphate Attenuates Atherosclerosis in ApoE Knockout Mice Involving Cellular Regulation of the Inflammatory Response.Thromb Haemost. 2018 Jul;118(7):1329-1339. doi: 10.1055/s-0038-1657753. Epub 2018 Jun 6. Thromb Haemost. 2018. PMID: 29874688 Free PMC article.
-
Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice.Atherosclerosis. 2017 Jul;262:39-50. doi: 10.1016/j.atherosclerosis.2017.05.003. Epub 2017 May 5. Atherosclerosis. 2017. PMID: 28500865
-
Efficacy of Terpenoid in Attenuating Aortic Atherosclerosis in Apolipoprotein-E Deficient Mice: A Meta-Analysis of Animal Studies.Biomed Res Int. 2019 Jul 17;2019:2931831. doi: 10.1155/2019/2931831. eCollection 2019. Biomed Res Int. 2019. PMID: 31392210 Free PMC article.
-
Targeting Foam Cell Formation in Atherosclerosis: Therapeutic Potential of Natural Products.Pharmacol Rev. 2019 Oct;71(4):596-670. doi: 10.1124/pr.118.017178. Pharmacol Rev. 2019. PMID: 31554644 Review.
Cited by
-
Tanshinol borneol ester on nanostructured lipid carriers has longer brain and systemic effector retention and better antioxidant activity in vivo.Int J Nanomedicine. 2018 Apr 12;13:2265-2274. doi: 10.2147/IJN.S159789. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29695905 Free PMC article.
-
Isoborneol Attenuates Low-Density Lipoprotein Accumulation and Foam Cell Formation in Macrophages.Drug Des Devel Ther. 2020 Jan 15;14:167-173. doi: 10.2147/DDDT.S233013. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32021101 Free PMC article.
-
The Signaling Pathways Involved in the Antiatherosclerotic Effects Produced by Chinese Herbal Medicines.Biomed Res Int. 2018 Jun 13;2018:5392375. doi: 10.1155/2018/5392375. eCollection 2018. Biomed Res Int. 2018. PMID: 30009170 Free PMC article. Review.
-
The Opportunities and Challenges of Peroxisome Proliferator-Activated Receptors Ligands in Clinical Drug Discovery and Development.Int J Mol Sci. 2018 Jul 27;19(8):2189. doi: 10.3390/ijms19082189. Int J Mol Sci. 2018. PMID: 30060458 Free PMC article. Review.
-
PPAR-Mediated Toxicology and Applied Pharmacology.Cells. 2020 Feb 3;9(2):352. doi: 10.3390/cells9020352. Cells. 2020. PMID: 32028670 Free PMC article. Review.
References
-
- Back M, Hansson GK. Anti‐inflammatory therapies for atherosclerosis. Nat Rev Cardiol. 2015;12:199–211. - PubMed
-
- Nahrendorf M, Swirski FK. Immunology neutrophil‐macrophage communication in inflammation and atherosclerosis. Science. 2015;349:237–238. - PubMed
-
- Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11:276–289. - PubMed
-
- Khan R, Spagnoli V, Tardif JC, L'Allier PL. Novel anti‐inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis. 2015;240:497–509. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

