Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis
- PMID: 28972001
- PMCID: PMC5973494
- DOI: 10.1161/CIRCULATIONAHA.117.028069
Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis
Abstract
Background: An emerging metabolic theory of pulmonary hypertension (PH) suggests that cellular and mitochondrial metabolic dysfunction underlies the pathology of this disease. We and others have previously demonstrated the existence of hyperproliferative, apoptosis-resistant, proinflammatory adventitial fibroblasts from human and bovine hypertensive pulmonary arterial walls (PH-Fibs) that exhibit constitutive reprogramming of glycolytic and mitochondrial metabolism, accompanied by an increased ratio of glucose catabolism through glycolysis versus the tricarboxylic acid cycle. However, the mechanisms responsible for these metabolic alterations in PH-Fibs remain unknown. We hypothesized that in PH-Fibs microRNA-124 (miR-124) regulates PTBP1 (polypyrimidine tract binding protein 1) expression to control alternative splicing of pyruvate kinase muscle (PKM) isoforms 1 and 2, resulting in an increased PKM2/PKM1 ratio, which promotes glycolysis and proliferation even in aerobic environments.
Methods: Pulmonary adventitial fibroblasts were isolated from calves and humans with severe PH (PH-Fibs) and from normal subjects. PTBP1 gene knockdown was achieved via PTBP1-siRNA; restoration of miR-124 was performed with miR-124 mimic. TEPP-46 and shikonin were used to manipulate PKM2 glycolytic function. Histone deacetylase inhibitors were used to treat cells. Metabolic products were determined by mass spectrometry-based metabolomics analyses, and mitochondrial function was analyzed by confocal microscopy and spectrofluorometry.
Results: We detected an increased PKM2/PKM1 ratio in PH-Fibs compared with normal subjects. PKM2 inhibition reversed the glycolytic status of PH-Fibs, decreased their cell proliferation, and attenuated macrophage interleukin-1β expression. Furthermore, normalizing the PKM2/PKM1 ratio in PH-Fibs by miR-124 overexpression or PTBP1 knockdown reversed the glycolytic phenotype (decreased the production of glycolytic intermediates and byproducts, ie, lactate), rescued mitochondrial reprogramming, and decreased cell proliferation. Pharmacological manipulation of PKM2 activity with TEPP-46 and shikonin or treatment with histone deacetylase inhibitors produced similar results.
Conclusions: In PH, miR-124, through the alternative splicing factor PTBP1, regulates the PKM2/PKM1 ratio, the overall metabolic, proliferative, and inflammatory state of cells. This PH phenotype can be rescued with interventions at various levels of the metabolic cascade. These findings suggest a more integrated view of vascular cell metabolism, which may open unique therapeutic prospects in targeting the dynamic glycolytic and mitochondrial interactions and between mesenchymal inflammatory cells in PH.
Keywords: TEEP-46; hypoxia; metabolism; mitochondria; pyruvate kinase; shikonin; splicing factors.
© 2017 American Heart Association, Inc.
Figures
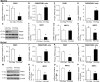




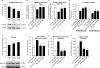


Comment in
-
Pyruvate Kinase and Warburg Metabolism in Pulmonary Arterial Hypertension: Uncoupled Glycolysis and the Cancer-Like Phenotype of Pulmonary Arterial Hypertension.Circulation. 2017 Dec 19;136(25):2486-2490. doi: 10.1161/CIRCULATIONAHA.117.031655. Circulation. 2017. PMID: 29255124 Free PMC article. No abstract available.
References
-
- Humbert M, Lau EM, Montani D, Jais X, Sitbon O, Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014;130:2189–2208. - PubMed
-
- Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational Advances in the Field of Pulmonary Hypertension. From Cancer Biology to New Pulmonary Arterial Hypertension Therapeutics. Targeting Cell Growth and Proliferation Signaling Hubs. Am J Respir Crit Care Med. 2017;195:425–437. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

