Interphotoreceptor retinoid-binding protein removes all- trans-retinol and retinal from rod outer segments, preventing lipofuscin precursor formation
- PMID: 28972139
- PMCID: PMC5702674
- DOI: 10.1074/jbc.M117.795187
Interphotoreceptor retinoid-binding protein removes all- trans-retinol and retinal from rod outer segments, preventing lipofuscin precursor formation
Abstract
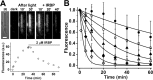
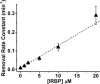
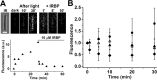
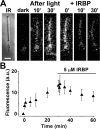
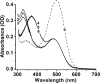
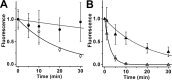
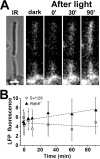
Interphotoreceptor retinoid-binding protein (IRBP) is a specialized lipophilic carrier that binds the all-trans and 11-cis isomers of retinal and retinol, and this facilitates their transport between photoreceptors and cells in the retina. One of these retinoids, all-trans-retinal, is released in the rod outer segment by photoactivated rhodopsin after light excitation. Following its release, all-trans-retinal is reduced by the retinol dehydrogenase RDH8 to all-trans-retinol in an NADPH-dependent reaction. However, all-trans-retinal can also react with outer segment components, sometimes forming lipofuscin precursors, which after conversion to lipofuscin accumulate in the lysosomes of the retinal pigment epithelium and display cytotoxic effects. Here, we have imaged the fluorescence of all-trans-retinol, all-trans-retinal, and lipofuscin precursors in real time in single isolated mouse rod photoreceptors. We found that IRBP removes all-trans-retinol from individual rod photoreceptors in a concentration-dependent manner. The rate constant for retinol removal increased linearly with IRBP concentration with a slope of 0.012 min-1 μm-1 IRBP also removed all-trans-retinal, but with much less efficacy, indicating that the reduction of retinal to retinol promotes faster clearance of the photoisomerized rhodopsin chromophore. The presence of physiological IRBP concentrations in the extracellular medium resulted in lower levels of all-trans-retinal and retinol in rod outer segments following light exposure. It also prevented light-induced lipofuscin precursor formation, but it did not remove precursors that were already present. These findings reveal an important and previously unappreciated role of IRBP in protecting the photoreceptor cells against the cytotoxic effects of accumulated all-trans-retinal.
Keywords: fluorescence; photoreceptor; retina; retinoid-binding protein; retinol.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

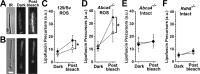
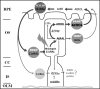
Similar articles
-
Interphotoreceptor retinoid-binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments.Biochemistry. 2007 Jul 24;46(29):8669-79. doi: 10.1021/bi7004619. Epub 2007 Jun 30. Biochemistry. 2007. PMID: 17602665 Free PMC article.
-
All-trans retinal levels and formation of lipofuscin precursors after bleaching in rod photoreceptors from wild type and Abca4-/- mice.Exp Eye Res. 2017 Feb;155:121-127. doi: 10.1016/j.exer.2017.02.007. Epub 2017 Feb 17. Exp Eye Res. 2017. PMID: 28219732 Free PMC article.
-
Interphotoreceptor retinoid-binding protein (IRBP) promotes the release of all-trans retinol from the isolated retina following rhodopsin bleaching illumination.Exp Eye Res. 2005 Oct;81(4):455-63. doi: 10.1016/j.exer.2005.03.005. Epub 2005 Jun 2. Exp Eye Res. 2005. PMID: 15935345
-
The 11-cis Retinal Origins of Lipofuscin in the Retina.Prog Mol Biol Transl Sci. 2015;134:e1-12. doi: 10.1016/bs.pmbts.2015.07.022. Prog Mol Biol Transl Sci. 2015. PMID: 26310175 Review.
-
Interphotoreceptor retinoid-binding protein (IRBP). Molecular biology and physiological role in the visual cycle of rhodopsin.Mol Neurobiol. 1993 Spring;7(1):61-85. doi: 10.1007/BF02780609. Mol Neurobiol. 1993. PMID: 8318167 Review.
Cited by
-
Photooxidation mediated by 11-cis and all-trans retinal in single isolated mouse rod photoreceptors.Photochem Photobiol Sci. 2020 Oct 14;19(10):1300-1307. doi: 10.1039/d0pp00060d. Photochem Photobiol Sci. 2020. PMID: 32812970 Free PMC article.
-
Correcting QUEST Magnetic Resonance Imaging-Sensitive Free Radical Production in the Outer Retina In Vivo Does Not Correct Reduced Visual Performance in 24-Month-Old C57BL/6J Mice.Invest Ophthalmol Vis Sci. 2021 May 3;62(6):24. doi: 10.1167/iovs.62.6.24. Invest Ophthalmol Vis Sci. 2021. PMID: 34036313 Free PMC article.
-
Stargardt's Disease: Molecular Pathogenesis and Current Therapeutic Landscape.Int J Mol Sci. 2025 Jul 21;26(14):7006. doi: 10.3390/ijms26147006. Int J Mol Sci. 2025. PMID: 40725253 Free PMC article. Review.
-
Preventing diabetic retinopathy by mitigating subretinal space oxidative stress in vivo.Vis Neurosci. 2020 Jun 15;37:E002. doi: 10.1017/S0952523820000024. Vis Neurosci. 2020. PMID: 32536351 Free PMC article. Review.
-
Novel fluorescent GPCR biosensor detects retinal equilibrium binding to opsin and active G protein and arrestin signaling conformations.J Biol Chem. 2020 Dec 18;295(51):17486-17496. doi: 10.1074/jbc.RA120.014631. J Biol Chem. 2020. PMID: 33453993 Free PMC article.
References
-
- Ebrey T., and Koutalos Y. (2001) Vertebrate photoreceptors. Prog. Retin. Eye Res. 20, 49–94 - PubMed
-
- Wald G. (1968) Molecular basis of visual excitation. Science 162, 230–239 - PubMed
-
- Rattner A., Smallwood P. M., and Nathans J. (2000) Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J. Biol. Chem. 275, 11034–11043 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

